Content table
Introduction
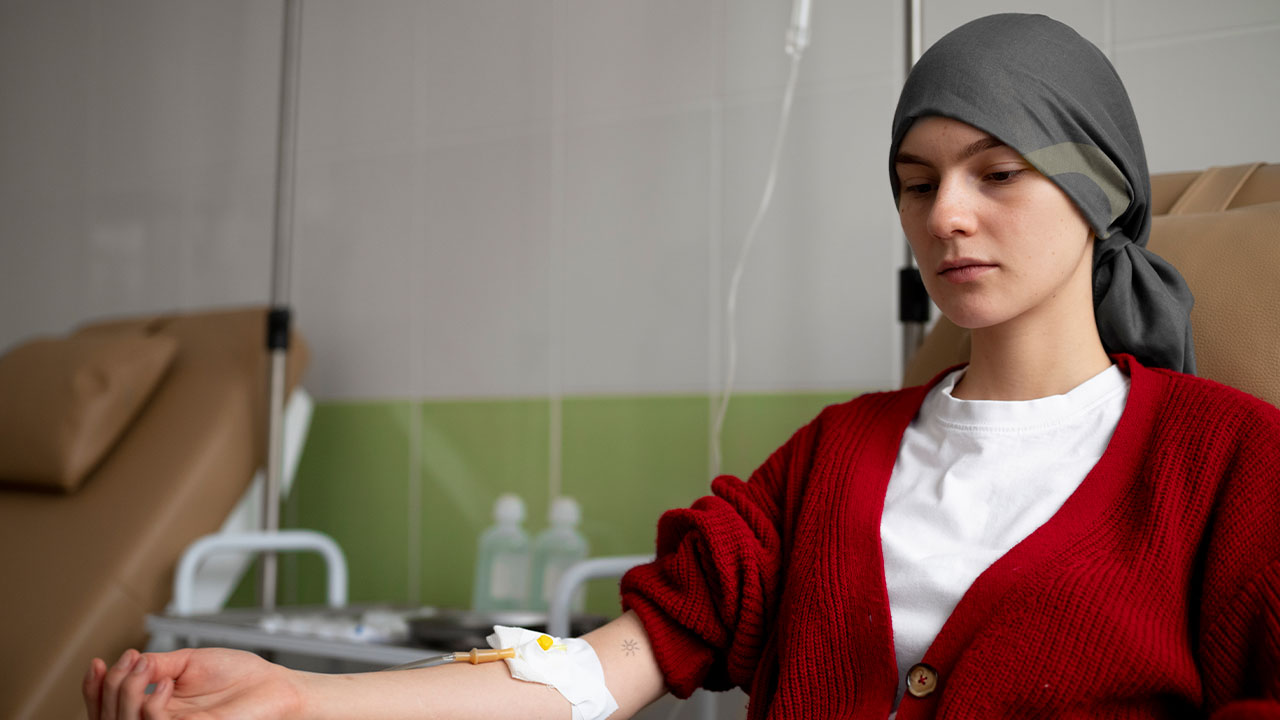
Infertility, the inability to conceive after a year or more of regular unprotected intercourse, affects millions of individuals and couples worldwide. This condition can be emotionally and psychologically demanding, leading to feelings of frustration, inadequacy, and profound stress. The World Health Organization (WHO) estimates that between 48 million couples and 186 million individuals live with infertility globally, making it a significant public health concern. The causes of infertility are multifaceted, encompassing a wide range of physiological, environmental, and lifestyle factors. In women, infertility is often linked to issues such as ovulatory disorders, fallopian tube damage, endometriosis, or uterine abnormalities. For men, it may be due to problems with sperm production, function, or delivery. Additionally, a significant portion of infertility cases remain unexplained, further complicating diagnosis and treatment.
1-B. Role of Stem Cells in Modern Medicine
In reproductive medicine, stem cells are being explored for their potential to regenerate damaged ovarian tissue, improve egg quality, and even restore spermatogenesis in men with infertility. For women with diminished ovarian reserves, stem cell therapy could offer a way to rejuvenate the ovaries, potentially increasing the chances of conception. In men, stem cells could be used to generate healthy sperm cells in cases where traditional treatments have failed. Moreover, stem cells could play a critical role in improving the outcomes of assisted reproductive technologies (ART) such as IVF. By enhancing the quality of eggs or sperm, or by creating a more conducive environment in the uterus, stem cells could increase the success rates of these procedures, offering new hope to those struggling with infertility.
The use of stem cells in treating infertility is still in its early stages, with much of the research being conducted in laboratory settings or clinical trials. However, the preliminary results are promising, suggesting that stem cell therapy could become a standard option in the fertility treatment arsenal in the near future. As research progresses, it is likely that we will see more refined and targeted applications of stem cells in reproductive medicine, potentially transforming the landscape of infertility treatment.
In summary, the integration of stem cells into reproductive medicine represents a revolutionary advance with the potential to significantly improve the outcomes for individuals and couples facing infertility. As research continues to unfold, stem cell therapy could offer a new paradigm in the treatment of infertility, addressing the root causes of reproductive issues and providing renewed hope for many.
Understanding Stem Cells
2-A. What Are Stem Cells
Stem cells have emerged as a pivotal tool in advancing medical treatments across a broad spectrum of diseases and conditions, including infertility. Their unique ability to differentiate into various cell types offers promising avenues for repairing or replacing damaged tissues and organs. In the context of reproductive health, stem cells hold the potential to address some of the underlying causes of infertility that have traditionally been challenging to treat.
2-B. Types of Stem Cells
Stem cells can be classified into several types, including embryonic stem cells, adult stem cells, and induced pluripotent stem cells (iPSCs). Each type has distinct characteristics and potential applications in medicine. For example, embryonic stem cells, derived from early-stage embryos, have the ability to differentiate into any cell type in the body, making them highly versatile for therapeutic purposes. Adult stem cells, found in various tissues such as bone marrow and fat, are more limited in their differentiation potential but are less controversial and more readily available. iPSCs, created by reprogramming adult cells to an embryonic-like state, combine the advantages of embryonic stem cells with reduced ethical concerns.
Infertility is a complex condition with a multitude of potential causes, affecting both men and women. Understanding these causes is crucial for diagnosing and effectively treating infertility. The most common causes can be broadly categorized into hormonal imbalances, structural issues, and genetic factors.
In Women:
- Hormonal Imbalances: Hormonal disorders are among the leading causes of female infertility. Conditions such as polycystic ovary syndrome (PCOS) can disrupt the regular release of eggs (ovulation), leading to irregular menstrual cycles or anovulation, where no egg is released. Other hormonal issues, such as thyroid disorders (hyperthyroidism or hypothyroidism) or hyperprolactinemia (elevated levels of prolactin), can also interfere with ovulation.
- Structural Issues: Structural abnormalities in the reproductive organs can significantly impact a woman’s fertility. Blockages in the fallopian tubes, often caused by pelvic inflammatory disease (PID), endometriosis, or previous surgeries, can prevent sperm from reaching the egg or block a fertilized egg from reaching the uterus. Uterine abnormalities, such as fibroids, polyps, or congenital malformations, can also affect the implantation of the embryo or lead to miscarriage.
- Genetic Factors: Genetic conditions can contribute to infertility by affecting the quality and function of the eggs or the reproductive organs. For instance, Turner syndrome, a condition where a woman is born with only one X chromosome, can lead to ovarian failure. Additionally, certain inherited mutations, such as those in the BRCA1 or BRCA2 genes, can impact fertility by increasing the risk of ovarian or breast cancer, which may require treatments that impair fertility.
In Men:
- Hormonal Imbalances: Just as in women, hormonal imbalances can lead to infertility in men. Low levels of testosterone, often caused by conditions such as hypogonadism, can result in a decreased production of sperm (oligospermia) or a complete lack of sperm in the ejaculate (azoospermia). Other hormonal issues, such as pituitary gland disorders, can also impair sperm production and function.
- Structural Issues: Structural problems in the male reproductive system can prevent the delivery of sperm. Varicoceles, which are enlarged veins within the scrotum, can affect sperm production and quality by increasing the temperature of the testes. Blockages in the vas deferens or epididymis, often due to infections, injuries, or congenital defects, can prevent sperm from being ejaculated. Additionally, conditions such as undescended testes (cryptorchidism) can impair sperm production if left untreated.
- Genetic Factors: Genetic abnormalities can also contribute to male infertility. For example, Klinefelter syndrome, where a man has an extra X chromosome (XXY instead of XY), can result in reduced testosterone levels and poor sperm production. Other genetic mutations, such as deletions in the Y chromosome, can lead to severe oligospermia or azoospermia. Additionally, cystic fibrosis, a genetic disorder that primarily affects the lungs, can also cause congenital absence of the vas deferens, leading to obstructive azoospermia.
3-A. Unexplained Infertility
Unexplained infertility is a diagnosis given when no clear cause can be identified after a thorough evaluation of both partners. This condition accounts for approximately 10-20% of all infertility cases and presents significant challenges for both patients and healthcare providers.
One of the main difficulties with unexplained infertility is that it leaves couples without a specific cause to address, making it harder to develop a targeted treatment plan. Despite normal test results for ovulation, sperm quality, and tubal patency, these couples still struggle to conceive. This lack of a clear diagnosis can lead to frustration and anxiety, as the path forward is often unclear.
There are several theories as to why infertility might remain unexplained. Some experts believe that subtle issues, such as minor hormonal imbalances, immune system disorders, or undetected genetic factors, could be at play but are not easily identified with standard diagnostic tests. Additionally, problems with the interaction between the sperm and the egg, or issues with embryo implantation, might be responsible but are difficult to detect with current technology.
For couples diagnosed with unexplained infertility, treatment typically involves a combination of approaches, such as lifestyle modifications, ovulation induction, intrauterine insemination (IUI), or IVF. However, the lack of a specific cause often means that these treatments are less predictable in their outcomes compared to cases where a clear cause has been identified.
Unexplained infertility highlights the limitations of current diagnostic tools and the need for continued research into the underlying causes of infertility. As medical science advances, it is hoped that more of these “unexplained” cases will be resolved, leading to more effective treatments and better outcomes for affected couples.
Current Infertility Treatments
4-A. IVF (In Vitro Fertilization)
In vitro fertilization (IVF) is one of the most widely used and effective treatments for infertility, helping many couples achieve pregnancy when other methods have failed. IVF involves a series of complex steps designed to fertilize an egg outside the woman’s body and implant the resulting embryo into the uterus.
The IVF process begins with ovarian stimulation, where a woman receives hormone injections to stimulate her ovaries to produce multiple eggs in one cycle. This is crucial because in a natural cycle, a woman typically releases only one egg. By producing multiple eggs, IVF increases the chances of fertilization and successful pregnancy. The hormone injections usually include follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which promote the development of multiple follicles in the ovaries. Once the eggs have matured, they are retrieved from the ovaries in a minor surgical procedure known as egg retrieval. This procedure is typically done under sedation and involves using an ultrasound-guided needle to extract the eggs from the ovarian follicles. The eggs are then immediately taken to the laboratory for fertilization. In the laboratory, the retrieved eggs are combined with sperm from the male partner or a donor in a controlled environment. Fertilization can occur naturally by placing the eggs and sperm together in a culture medium, or it can be assisted using a technique called intracytoplasmic sperm injection (ICSI), where a single sperm is directly injected into an egg. ICSI is often used in cases where there are significant issues with sperm quality, such as low motility or abnormal morphology. After fertilization, the resulting embryos are cultured in the laboratory for several days, typically three to five, until they reach a stage where they can be implanted into the uterus. The healthiest embryos are selected for embryo transfer, which is a simple procedure where the embryos are placed into the woman’s uterus using a thin catheter. Any remaining high-quality embryos can be frozen for future use.
The success rate of IVF varies based on several factors, including the age of the woman, the quality of the eggs and sperm, and the underlying cause of infertility. On average, the success rate of IVF per cycle is around 20-35%, with younger women generally having higher success rates. However, success rates decrease with age, particularly after the age of 35. Despite its success, IVF is not without limitations and challenges. The process can be emotionally and physically demanding, requiring multiple injections, frequent monitoring, and invasive procedures. Additionally, IVF can be expensive, with each cycle costing thousands of dollars, and it may take several cycles to achieve a successful pregnancy. There are also risks associated with IVF, such as ovarian hyperstimulation syndrome (OHSS), multiple pregnancies (twins, triplets, etc.), and the potential for ectopic pregnancy.
4-B. Hormonal Treatments
Hormonal treatments are another cornerstone of infertility therapy, used to regulate or stimulate reproductive functions in both men and women. These treatments can address various hormonal imbalances that interfere with ovulation, sperm production, and other aspects of fertility.
In women, hormonal therapy is often used to induce or regulate ovulation, particularly in cases where irregular or absent ovulation is the primary cause of infertility. Common medications include Clomiphene Citrate (Clomid), which stimulates the release of hormones necessary for ovulation, and Gonadotropins, which are injectable hormones that directly stimulate the ovaries to produce multiple eggs. Gonadotropins include FSH and LH, which are also used in IVF cycles to promote the growth of ovarian follicles.
For women with conditions like polycystic ovary syndrome (PCOS), which is characterized by irregular ovulation and elevated levels of androgens (male hormones), anti-androgens and insulin-sensitizing agents such as Metformin may be prescribed to restore normal ovulatory cycles. In cases of luteal phase defects, where the second half of the menstrual cycle is insufficient to support pregnancy, progesterone supplements may be given to enhance the uterine lining and improve the chances of embryo implantation.
In men, hormonal treatments may be used to address issues such as low testosterone levels, which can impair sperm production. Human chorionic gonadotropin (hCG) injections are often prescribed to stimulate the testes to produce more testosterone and sperm. Additionally, medications such as gonadotropin-releasing hormone (GnRH) analogs can be used to treat conditions like hypogonadotropic hypogonadism, where there is insufficient stimulation of the testes due to a lack of GnRH from the brain.
While hormonal treatments can be highly effective, they also come with potential side effects and risks. In women, side effects can include hot flashes, mood swings, headaches, and an increased risk of multiple pregnancies. Long-term use of certain hormones can also increase the risk of ovarian hyperstimulation syndrome (OHSS) and other complications. In men, side effects may include weight gain, mood changes, and, in some cases, a temporary decrease in sperm production before improvement is seen.
4-C. Surgery
Surgical interventions play a crucial role in the treatment of certain types of infertility, particularly when structural abnormalities or blockages are the underlying cause. Advances in minimally invasive surgery have greatly improved the outcomes and recovery times for these procedures.
Laparoscopic surgery is commonly used in women to diagnose and treat conditions such as endometriosis, a disorder where tissue similar to the lining of the uterus grows outside the uterine cavity, causing pain and infertility. During laparoscopy, a small incision is made in the abdomen, and a thin camera (laparoscope) is inserted to view the pelvic organs. Endometriosis lesions can be excised or ablated during the same procedure, which can improve fertility outcomes by restoring normal pelvic anatomy.
Laparoscopy is also used to treat ovarian cysts, fibroids, and tubal blockages, all of which can impair fertility. For instance, in cases where the fallopian tubes are blocked or damaged, tubal surgery may be performed to remove the blockage or repair the tubes, allowing sperm to reach the egg naturally.
In men, surgical treatment of infertility often involves procedures to correct varicocele, a condition where enlarged veins in the scrotum increase the temperature of the testes, potentially leading to reduced sperm production and quality. Varicocelectomy, the surgical removal of these varicose veins, can improve sperm parameters and increase the chances of natural conception.
Another common surgical intervention in men is vasectomy reversal, which can restore fertility in men who have previously undergone a vasectomy. This microsurgical procedure reconnects the vas deferens, allowing sperm to be present in the ejaculate once again.
In cases where structural issues are too severe for repair or where previous surgeries have failed, assisted reproductive technologies (ART) such as IVF may be recommended as the next step. However, surgical interventions remain a vital option for many individuals and couples, offering the potential to correct underlying issues and restore natural fertility.
Surgical treatments, while often effective, carry risks such as infection, bleeding, and damage to surrounding organs. The decision to undergo surgery is typically made after careful consideration of the potential benefits and risks, often in consultation with a reproductive specialist.
The Role of Stem Cells in Reproductive Medicine
5-A. Stem Cells and Reproductive Health
Stem cells have emerged as a transformative tool in modern medicine, with their unique ability to differentiate into various cell types, offering immense potential in the field of reproductive health. In reproductive medicine, stem cells are being explored for their capacity to repair, regenerate, and rejuvenate damaged or dysfunctional reproductive tissues, which can significantly impact fertility.
One of the primary ways stem cells contribute to reproductive health is through the regeneration of ovarian tissue. In women, aging, chemotherapy, radiation, and certain medical conditions can lead to premature ovarian failure or diminished ovarian reserve, where the quantity and quality of eggs are reduced. Stem cell therapy offers the potential to reverse this damage by promoting the regeneration of healthy ovarian tissue. Mesenchymal stem cells (MSCs), for example, have shown promise in preclinical studies for their ability to repair ovarian tissue, enhance follicle development, and even restore hormone production, which is critical for ovulation and overall reproductive function.
In addition to ovarian tissue regeneration, stem cells may also play a role in improving uterine health. The endometrium, the lining of the uterus, is essential for embryo implantation and successful pregnancy. Conditions like Asherman’s syndrome, characterized by scar tissue formation in the uterus, can severely impair fertility. Stem cells, particularly endometrial stem cells, have been investigated for their potential to regenerate and repair the endometrial lining, thereby improving the chances of embryo implantation and reducing the risk of miscarriage.
In men, stem cells are being studied for their ability to restore spermatogenesis, the process by which sperm are produced. Infertility in men can result from various factors, including genetic disorders, environmental toxins, and medical treatments that damage the testes. Stem cells, such as spermatogonial stem cells (SSCs), have shown the potential to restore sperm production by regenerating the germ cells that give rise to sperm. This could be particularly beneficial for men who have become infertile due to cancer treatments, as SSC transplantation has the potential to reinitiate the production of viable sperm, offering a path to biological parenthood.
Stem cells also hold promise for addressing unexplained infertility, where the cause of infertility is not identifiable through conventional diagnostic methods. By rejuvenating reproductive tissues and improving the overall health of the reproductive system, stem cells could enhance fertility even in cases where traditional treatments have failed.
5-B. Advantages of Using Stem Cells in Treating Infertility
The use of stem cells in treating infertility offers several potential advantages that make it a promising area of research and application in reproductive medicine. One of the most significant benefits is the ability of stem cells to improve treatment outcomes for individuals and couples facing infertility.
For patients who have exhausted conventional treatments, stem cell therapy represents a new frontier, offering hope where other methods have not succeeded. For instance, women with premature ovarian failure or diminished ovarian reserve often have limited options with traditional fertility treatments. Stem cell therapy could potentially rejuvenate ovarian function, improving the chances of natural conception or increasing the effectiveness of assisted reproductive technologies (ART) like in vitro fertilization (IVF).
Another advantage of stem cell therapy is its potential to address the root causes of infertility rather than just managing the symptoms. Traditional fertility treatments often focus on stimulating ovulation or improving sperm count without addressing underlying tissue damage or dysfunction. Stem cells, however, have the potential to repair and regenerate the reproductive organs themselves, offering a more comprehensive approach to treating infertility. Furthermore, stem cells could reduce the need for invasive procedures and repeated cycles of ART. For example, if stem cell therapy successfully restores ovarian function or regenerates the endometrial lining, patients might not need to undergo multiple rounds of IVF or other intensive treatments. This not only reduces physical and emotional stress but also lowers the financial burden associated with fertility treatments. Stem cell therapy also holds the promise of personalized medicine in reproductive health. As research advances, it may become possible to develop stem cell treatments tailored to an individual’s specific reproductive challenges, whether that involves regenerating ovarian tissue, restoring spermatogenesis, or repairing uterine damage. Personalized stem cell therapy could lead to higher success rates and better overall outcomes, providing a more effective solution for those struggling with infertility.
While stem cell therapy for infertility is still largely in the experimental stage, the early results are encouraging, and ongoing research continues to unlock new possibilities. As this field advances, stem cells may become an integral part of the reproductive medicine toolkit, offering new hope to countless individuals and couples seeking to overcome infertility.
Stem Cells in Male Infertility Treatment
6-A. Restoring Spermatogenesis
Spermatogenesis, the process by which sperm cells are produced in the testes, is crucial for male fertility. However, various factors, including genetic abnormalities, environmental toxins, infections, and medical treatments like chemotherapy, can impair this process, leading to male infertility. In cases where traditional treatments such as hormonal therapy or surgical intervention fail to restore sperm production, stem cell therapy offers a promising alternative by directly targeting the underlying issues in spermatogenesis.
Stem cells, particularly spermatogonial stem cells (SSCs), are the foundation of spermatogenesis. These stem cells reside in the seminiferous tubules of the testes and are responsible for continuously dividing and differentiating into mature sperm throughout a man’s reproductive life. When SSCs are damaged or depleted, spermatogenesis can be severely compromised, resulting in reduced sperm count or complete absence of sperm (azoospermia). Stem cell therapy aims to restore spermatogenesis by either transplanting healthy SSCs into the testes or stimulating the body’s existing stem cells to regenerate sperm-producing cells. SSC transplantation involves harvesting these stem cells from the patient or a donor and then reintroducing them into the testes. The transplanted SSCs are expected to repopulate the seminiferous tubules and resume the production of healthy sperm. This approach is particularly promising for men who have become infertile due to treatments like chemotherapy, which can destroy the existing SSCs but leave the surrounding environment conducive to stem cell regeneration.
Another approach involves using induced pluripotent stem cells (iPSCs), which are adult cells reprogrammed to an embryonic-like state, allowing them to differentiate into any cell type, including SSCs. In laboratory settings, iPSCs can be generated from a patient’s cells and then differentiated into SSCs, which can be transplanted back into the testes. This method not only addresses the issue of sperm production but also mitigates the risk of immune rejection since the cells are derived from the patient’s tissue.
Research into using mesenchymal stem cells (MSCs) for male infertility is also gaining momentum. MSCs, which are multipotent stromal cells found in bone marrow, adipose tissue, and other locations, have shown potential in supporting the regeneration of testicular tissue. When introduced into the testes, MSCs can create a supportive environment that enhances the survival and function of SSCs, promoting the regeneration of spermatogenesis.
The potential of stem cells to restore spermatogenesis represents a significant breakthrough in male infertility treatment. Unlike conventional therapies that often only manage symptoms, stem cell therapy targets the root cause by regenerating the cells necessary for sperm production. While this approach is still in the experimental stage, early results are promising, offering new hope to men who previously had limited or no options for restoring their fertility. Several case studies and clinical trials have demonstrated the potential of stem cell therapy to treat male infertility, particularly in cases where traditional methods have failed.
Stem Cells in Female Infertility Treatment
7-A. Improving Egg Quality
Egg quality is a critical factor in female fertility, with a direct impact on the chances of conception and the health of the pregnancy. As women age, the quality and quantity of their eggs naturally decline, leading to increased difficulty in achieving pregnancy and a higher risk of miscarriage. In addition to aging, other factors such as environmental toxins, medical treatments like chemotherapy, and certain genetic conditions can further compromise egg quality.
Stem cell therapy offers a promising avenue for improving egg quality in women facing infertility. Research has shown that certain types of stem cells, particularly mesenchymal stem cells (MSCs) and induced pluripotent stem cells (iPSCs), have the potential to enhance the viability and function of oocytes (egg cells). These stem cells can be introduced into the ovaries, where they may promote the rejuvenation of aging or damaged eggs by enhancing the cellular environment and providing essential growth factors that support oocyte development.
One of the key mechanisms by which stem cells improve egg quality is through their paracrine effects—the release of bioactive molecules that influence surrounding cells. MSCs secrete a variety of growth factors, cytokines, and exosomes that can reduce oxidative stress and promote the repair of DNA damage within oocytes. By mitigating oxidative damage, which is a major contributor to the decline in egg quality, stem cells can help preserve the genetic integrity of oocytes, thus improving their developmental potential.
Additionally, stem cells can enhance mitochondrial function within oocytes. Mitochondria, the energy-producing organelles in cells, play a crucial role in oocyte maturation and embryonic development. As women age, the efficiency of mitochondrial function in their eggs diminishes, leading to lower energy production and increased chances of chromosomal abnormalities. Stem cells, particularly iPSCs, can potentially restore mitochondrial function by either rejuvenating existing mitochondria or introducing healthy mitochondria into the oocytes, thereby improving their quality and viability.
7-B. Repairing Ovarian Tissue
Ovarian tissue damage, whether due to aging, medical treatments like chemotherapy, or certain diseases, can severely impair a woman’s fertility by reducing her ovarian reserve—the number of viable eggs available for fertilization. Stem cell therapy holds significant promise for repairing damaged ovarian tissue and restoring fertility in women who might otherwise have few or no options for conceiving.
The regenerative potential of stem cells, particularly bone marrow-derived mesenchymal stem cells (BM-MSCs) and adipose-derived stem cells (ADSCs), has been extensively studied for their ability to repair and regenerate ovarian tissue. These stem cells can be introduced into the ovaries, where they may contribute to the restoration of the ovarian microenvironment, promoting the survival and function of remaining follicles, or even initiating the formation of new follicles.
One of the ways stem cells repair ovarian tissue is through angiogenesis, the process of forming new blood vessels. By improving the blood supply to the ovaries, stem cells can enhance the delivery of oxygen and nutrients to the ovarian follicles, which is essential for their growth and maturation. Enhanced vascularization also helps remove metabolic waste and reduces the accumulation of harmful substances, creating a healthier environment for follicle development.
Stem cells also have the potential to regenerate ovarian stromal cells, which provide structural support and produce hormones necessary for follicle survival and oocyte maturation. By restoring the ovarian stroma, stem cells can help rejuvenate the overall ovarian function, potentially extending a woman’s fertile years and increasing her chances of natural conception or success with assisted reproductive technologies (ART).
7-C. Role in Uterine Health
The health of the uterus, particularly the endometrial lining, is crucial for successful embryo implantation and the maintenance of pregnancy. Conditions such as Asherman’s syndrome (intrauterine adhesions), chronic endometritis, and thin endometrium can severely impair the uterine environment, making it less receptive to embryo implantation and increasing the risk of miscarriage.
Stem cell therapy has emerged as a potential solution for improving uterine health and enhancing the receptivity of the endometrium to implanted embryos. Endometrial stem cells and MSCs are particularly promising in this regard due to their ability to regenerate the endometrial lining and repair damage caused by scarring or inflammation. In cases of Asherman’s syndrome, for example, where scar tissue formation within the uterus can prevent proper endometrial growth, stem cells can be used to promote the regeneration of healthy endometrial tissue. Studies have shown that introducing MSCs into the uterine cavity can help break down scar tissue and stimulate the growth of new, functional endometrial cells, restoring the normal thickness and receptivity of the uterine lining.
Stem cells also play a role in modulating the immune environment of the uterus. A balanced immune response is essential for successful implantation and the prevention of pregnancy loss. Stem cells can help regulate the immune cells in the endometrium, reducing inflammation and promoting a more favorable environment for embryo implantation. This immunomodulatory effect is particularly beneficial for women with chronic endometritis or those who have experienced recurrent implantation failure.
Moreover, stem cells can enhance the vascularization of the endometrium, ensuring that the uterine lining receives adequate blood supply to support embryo implantation and early pregnancy. Improved blood flow not only nourishes the growing embryo but also helps in the formation of a stable placenta, which is critical for sustaining the pregnancy.
By addressing these various aspects of uterine health, stem cell therapy holds the potential to significantly improve the chances of successful embryo implantation and reduce the risk of miscarriage in women with uterine factor infertility. As research continues to advance, stem cells may become an integral part of fertility treatments, offering new hope to women who struggle with conditions that compromise their uterine health.
Stem Cell Therapy for Endometriosis
8-A. Understanding Endometriosis
Endometriosis is a chronic and often painful condition in which tissue similar to the lining of the uterus, known as the endometrium, grows outside the uterine cavity. This ectopic endometrial tissue can be found on the ovaries, fallopian tubes, the outer surface of the uterus, and other organs within the pelvis. Endometriosis affects approximately 10-15% of women of reproductive age and is a significant cause of infertility.
The primary symptoms of endometriosis include severe menstrual cramps, chronic pelvic pain, pain during intercourse, and heavy menstrual bleeding. However, the condition’s impact on fertility is one of its most challenging aspects. Endometriosis can lead to infertility through several mechanisms:
- Tubal Blockage: The growth of endometrial tissue on or near the fallopian tubes can cause scarring and blockages, preventing the sperm from reaching the egg or the fertilized egg from reaching the uterus.
- Ovarian Dysfunction: Endometriosis can form cysts on the ovaries, known as endometriomas, which can damage ovarian tissue, reduce the ovarian reserve, and impair the release of eggs during ovulation.
- Inflammation: The chronic inflammation associated with endometriosis can create a hostile environment for sperm and eggs, affecting fertilization and embryo development.
- Altered Immune Response: Endometriosis can disrupt the normal immune response, leading to increased production of substances that may hinder embryo implantation or contribute to miscarriage.
Given its complex nature and the challenges it poses to fertility, endometriosis often requires a multifaceted treatment approach. While traditional treatments include hormonal therapies and surgery, these options may not be sufficient for all patients, particularly those with severe or recurrent endometriosis. This is where stem cell therapy presents a promising new avenue for treatment.
8-B. How Stem Cells Can Help
Stem cell therapy offers a novel approach to treating endometriosis by targeting the underlying causes of the disease rather than merely alleviating symptoms. The potential of stem cells to repair and regenerate damaged tissues, reduce inflammation, and modulate the immune system makes them a powerful tool in the fight against endometriosis-related infertility.
One of the primary ways stem cells can help is through tissue regeneration. Endometriosis often causes significant scarring and damage to reproductive tissues, particularly the ovaries and fallopian tubes. Stem cells, especially mesenchymal stem cells (MSCs), can migrate to sites of injury and promote the repair and regeneration of damaged tissue. MSCs can differentiate into various cell types, including those needed to rebuild healthy ovarian and tubal tissues, thereby restoring normal function and improving fertility.
In addition to tissue regeneration, stem cells have potent anti-inflammatory properties. Endometriosis is characterized by chronic inflammation, which not only contributes to pain but also creates an unfavorable environment for conception. MSCs can reduce inflammation by secreting anti-inflammatory cytokines and growth factors that help to calm the immune response, reduce the production of harmful inflammatory substances, and promote a more conducive environment for pregnancy.
Stem cells also play a role in immune modulation. The altered immune response seen in endometriosis can impair embryo implantation and increase the risk of miscarriage. Stem cells can help regulate the immune system, reducing the production of antibodies and immune cells that attack the endometrial tissue and instead promoting a more balanced and supportive immune environment for embryo development and implantation.
Furthermore, stem cells can potentially prevent the recurrence of endometriosis after surgical treatment. Recurrence is a common issue with endometriosis, as even after surgical removal of endometrial lesions, the disease can return. Stem cells can be used in conjunction with surgery to promote the healing of the affected areas and reduce the likelihood of new lesions forming.
Stem Cell Therapy and IVF Success Rates
9-A. Enhancing IVF Success with Stem Cells
The success rates of IVF can vary significantly, depending on factors such as the age of the woman, the quality of the eggs and sperm, and the overall health of the reproductive system. Despite advances in IVF techniques, challenges such as poor embryo quality, implantation failure, and ovarian insufficiency continue to limit its effectiveness for many patients.
Stem cell therapy has emerged as a promising approach to enhance the success rates of IVF by addressing some of the underlying issues that contribute to its limitations. By improving the quality of eggs, rejuvenating the ovarian environment, and enhancing uterine receptivity, stem cells can potentially increase the likelihood of successful embryo implantation and pregnancy.
One of the primary ways stem cells can enhance IVF outcomes is by improving egg quality. As women age or undergo treatments like chemotherapy, the quality and quantity of their eggs often decline, reducing the chances of successful fertilization and healthy embryo development. Stem cells, particularly mesenchymal stem cells (MSCs) and induced pluripotent stem cells (iPSCs), have shown potential in laboratory settings to rejuvenate aging oocytes by enhancing mitochondrial function, reducing oxidative stress, and repairing DNA damage. These improvements can lead to healthier eggs that are more likely to result in viable embryos, thus increasing the success rates of IVF. In addition to improving egg quality, stem cells can play a crucial role in rejuvenating the ovarian environment. Conditions such as diminished ovarian reserve or premature ovarian failure can severely limit the number of eggs available for IVF. Stem cell therapy aims to regenerate ovarian tissue, promote follicle development, and restore hormone production, thereby increasing the number of eggs that can be retrieved and fertilized during an IVF cycle. Early studies have shown that stem cells can stimulate the growth of new follicles in animal models, and similar approaches are being explored in clinical trials for women with low ovarian reserves. Another critical aspect of IVF success is the receptivity of the uterus to embryo implantation. Even when high-quality embryos are produced, implantation failure can occur if the endometrial lining of the uterus is not adequately prepared to receive the embryo. Stem cells, particularly endometrial stem cells, can help improve the thickness and vascularization of the endometrial lining, creating a more supportive environment for embryo implantation. By enhancing uterine receptivity, stem cells can reduce the likelihood of implantation failure and increase the chances of a successful pregnancy. Furthermore, stem cells may also help address recurrent implantation failure (RIF), a condition where embryos consistently fail to implant despite repeated IVF attempts. For women with RIF, stem cell therapy offers a novel approach by potentially repairing underlying endometrial defects, modulating the immune response, and improving the overall uterine environment, thus increasing the chances of successful implantation in subsequent IVF cycles.
9-B. Comparative Studies
Several comparative studies have been conducted to evaluate the effectiveness of integrating stem cell therapy with traditional IVF treatments. These studies aim to determine whether the addition of stem cells can significantly improve the success rates of IVF, particularly for patients who have experienced repeated IVF failures or who have poor prognoses due to factors like advanced maternal age or diminished ovarian reserve. One such study involved a group of women with diminished ovarian reserve, a condition characterized by a reduced number of viable eggs in the ovaries. The study compared the IVF success rates of women who received standard IVF treatment with those who received a combination of IVF and autologous stem cell therapy, where stem cells derived from the patient’s bone marrow were injected into the ovaries. The results showed that the women who received stem cell therapy had a higher number of retrieved eggs, improved embryo quality, and a significantly higher pregnancy rate compared to those who underwent standard IVF alone. Another study focused on women with recurrent implantation failure (RIF). In this study, patients were divided into two groups: one group received conventional IVF treatment, while the other group received IVF combined with stem cell therapy. The stem cell therapy involved the injection of endometrial stem cells to enhance uterine receptivity. The findings revealed that the group receiving stem cell therapy had a notably higher implantation rate and ongoing pregnancy rate compared to the control group, suggesting that stem cells can effectively address implantation issues that conventional IVF alone might not overcome.
In cases of poor egg quality, particularly in older women, comparative studies have explored the impact of stem cell therapy on egg rejuvenation. One such study used iPSCs derived from the patients’ somatic cells to rejuvenate aging oocytes before IVF. The study found that the embryos derived from the rejuvenated eggs had higher developmental potential and resulted in higher clinical pregnancy rates compared to embryos derived from untreated eggs.
While these studies are promising, it’s important to note that stem cell therapy in conjunction with IVF is still a developing field, and more extensive research and clinical trials are needed to fully understand its efficacy and safety. Nonetheless, the preliminary results suggest that stem cell therapy could become a valuable tool in enhancing IVF outcomes, particularly for patients who have struggled with infertility despite multiple IVF attempts. The integration of stem cell therapy into IVF protocols represents a significant advancement in reproductive medicine. By addressing the underlying issues that contribute to infertility, stem cells offer the potential to improve the success rates of IVF, providing new hope to couples facing challenging fertility issues. As research continues to progress, it is likely that stem cell therapy will play an increasingly important role in the future of assisted reproductive technologies.
Ethical Considerations and Challenges
10-A. Ethical Debates Surrounding Stem Cell Research
Stem cell research, particularly the use of embryonic stem cells, has been at the center of ethical debates since its inception. The primary ethical concern revolves around the source of these cells—human embryos. Embryonic stem cells are derived from embryos that are typically three to five days old, consisting of about 150 cells, and are at a stage called the blastocyst. These cells have the unique ability to develop into any type of cell in the human body, making them incredibly valuable for research and potential therapies. However, the process of harvesting these cells involves the destruction of the embryo, raising significant moral and ethical issues.
One of the main ethical arguments against the use of embryonic stem cells is the belief that human life begins at conception, and therefore, the destruction of an embryo is equivalent to taking a human life. This perspective is often rooted in religious and philosophical beliefs about the sanctity of human life. Opponents of embryonic stem cell research argue that no potential scientific or medical benefits can justify the destruction of embryos, as it violates the moral obligation to protect human life at all stages of development.
On the other hand, proponents of embryonic stem cell research emphasize the potential benefits of this research in treating a wide range of debilitating and life-threatening conditions, including infertility. They argue that the use of embryos, particularly those that are left over from in vitro fertilization (IVF) procedures and would otherwise be discarded, is ethically permissible if it leads to significant medical advances. The potential to cure diseases, regenerate damaged tissues, and alleviate suffering is seen as a compelling reason to pursue this line of research.
To address these ethical concerns, scientists have explored alternative sources of stem cells, such as induced pluripotent stem cells (iPSCs), which are adult cells reprogrammed to behave like embryonic stem cells. Since iPSCs do not require the destruction of embryos, they bypass the most contentious ethical issues. However, while iPSCs hold great promise, they are not yet a perfect substitute for embryonic stem cells, as there are differences in their capabilities and potential risks, such as genetic mutations that can occur during the reprogramming process.
The ethical debates surrounding stem cell research also extend to issues of consent and ownership. For example, there are concerns about how embryos used for research are obtained and whether donors fully understand the implications of their consent. Additionally, questions arise about the ownership and commercialization of stem cell lines, particularly when they are derived from human embryos.
10-B. Regulatory and Legal Challenges
The legal landscape governing stem cell therapy and research is complex and varies significantly across different countries and regions. These regulations are often shaped by the ethical considerations mentioned above, as well as by societal values, religious beliefs, and political climates.
In many countries, the use of embryonic stem cells is tightly regulated or even prohibited. For example, in the United States, federal funding for research involving the creation of new embryonic stem cell lines has been restricted since the early 2000s, though existing lines may be used. These restrictions have driven researchers to seek alternative sources of funding, often from private or state-level entities, which can lead to disparities in research opportunities and advancements. In contrast, countries like the United Kingdom have established regulatory frameworks that allow for embryonic stem cell research under strict conditions. The UK’s Human Fertilisation and Embryology Authority (HFEA) oversees research involving human embryos, ensuring that it is conducted ethically and with appropriate oversight. This regulatory approach balances the ethical concerns with the potential benefits of the research, allowing for scientific progress while maintaining public trust. However, the international nature of stem cell research poses additional regulatory challenges. Researchers often collaborate across borders, but differing legal frameworks can complicate these collaborations. For instance, a stem cell line created legally in one country may not be allowed to be imported or used in another due to differing regulations. This can hinder the sharing of resources and knowledge, slowing down the pace of scientific advancement. Moreover, the commercialization of stem cell therapies presents legal challenges related to intellectual property and patent rights. Companies and research institutions seek to protect their discoveries and innovations, but this can lead to disputes over who owns the rights to specific stem cell lines or therapies. Patenting stem cells also raises ethical questions, particularly when it comes to the commodification of human biological materials. Ensuring the safety and efficacy of stem cell therapies is another critical regulatory challenge. Stem cell treatments are still relatively new, and while they hold great promise, they also carry risks. Unproven and unregulated stem cell treatments have proliferated in some countries, leading to concerns about patient safety. Regulatory bodies must therefore establish rigorous standards for clinical trials and therapy approval, ensuring that only safe and effective treatments reach the market. The rapid pace of innovation in stem cell research further complicates regulatory oversight. As new techniques and therapies are developed, regulators must continually update their frameworks to address emerging ethical, legal, and safety issues. This requires a delicate balance between promoting innovation and protecting patients and ethical standards.
In conclusion, while stem cell research and therapy offer unprecedented potential in treating infertility and other conditions, they are accompanied by significant ethical and regulatory challenges. Addressing these challenges requires ongoing dialogue among scientists, ethicists, policymakers, and the public to develop frameworks that allow for responsible research and clinical applications while respecting moral and legal boundaries.
Risks and Potential Side Effects
11-A. Possible Complications of Stem Cell Therapy
Stem cell therapy holds significant promise in treating infertility, but like any medical treatment, it is not without risks and potential side effects. The complexity of stem cell treatments, which involve manipulating cells at a fundamental level, introduces various complications that need to be carefully considered.
One of the primary concerns is the risk of immune reactions. Although autologous stem cells (cells derived from the patient’s own body) are less likely to be rejected by the immune system, there is still a possibility of immune responses, particularly if the cells are manipulated extensively before being reintroduced. This could lead to inflammation, tissue damage, or even graft-versus-host disease (GVHD), a condition more commonly associated with organ transplants, where the transplanted cells attack the host’s tissues.
Another significant risk is the potential for tumor formation. Stem cells, especially pluripotent stem cells like embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), have the ability to divide and differentiate into various cell types. However, if these cells are not fully controlled, they could proliferate uncontrollably, leading to the development of tumors, including teratomas, which are tumors that can contain a variety of tissues such as hair, muscle, and bone. This risk is particularly concerning when using stem cells in reproductive tissues, where the environment might further promote abnormal growth.
Infections are another potential complication, particularly if the stem cells are not handled or administered under strictly sterile conditions. Contamination of stem cells during processing or storage could introduce pathogens into the patient’s body, leading to infections that could be difficult to treat, especially in reproductive organs.
Additionally, there is the risk of incomplete differentiation or incorrect cell integration. If stem cells do not fully differentiate into the desired cell type, they may not function as intended or might integrate improperly into the tissue. This could result in ineffective treatment or unexpected side effects. For instance, if stem cells intended to regenerate ovarian tissue do not differentiate correctly, they could fail to restore fertility or, worse, disrupt the normal function of the ovaries.
There are also concerns related to genetic mutations. The processes involved in creating induced pluripotent stem cells (iPSCs) involve reprogramming adult cells back to a pluripotent state, which can sometimes lead to genetic instability or mutations. These mutations might not only affect the effectiveness of the treatment but could also potentially lead to malignancies or other long-term health issues.
11-B. Long-Term Risks
While the short-term risks of stem cell therapy are better understood, the potential long-term risks remain a significant area of concern and ongoing study. One of the primary challenges is the lack of comprehensive long-term data, given that many stem cell therapies are relatively new and still in the experimental stages.
One potential long-term risk is the development of chronic conditions related to abnormal cell behavior. For example, if stem cells used to treat infertility do not integrate properly into the reproductive tissues, they could cause ongoing issues, such as chronic inflammation or fibrosis, which could affect fertility or overall reproductive health in the long term.
There is also the possibility that stem cells could migrate to unintended locations within the body. Once administered, stem cells might not stay confined to the target tissue and could travel to other organs, where they might differentiate into inappropriate cell types or disrupt normal function. This could lead to unforeseen complications, depending on where these cells migrate and what they become.
Moreover, the long-term genetic stability of stem cells remains a concern. Even if stem cells appear to function correctly initially, genetic mutations or epigenetic changes could accumulate over time, potentially leading to malignancies or other serious conditions years after the treatment. Continuous monitoring and long-term follow-up studies are necessary to understand these risks fully.
Immune system alterations are another potential long-term concern. Stem cell therapies, particularly those that involve immune modulation, could have lasting effects on the patient’s immune system. This could potentially lead to either increased susceptibility to infections or the development of autoimmune conditions, where the immune system begins to attack the body’s tissues.
Finally, the ethical and psychological implications of stem cell therapies should not be overlooked. Patients undergoing these treatments may face ongoing anxiety about the potential long-term effects, particularly in the absence of extensive long-term safety data. Additionally, the societal and ethical considerations of altering the body at a cellular level could have broader implications, especially if unforeseen consequences arise years or decades after treatment.
In summary, while stem cell therapy for infertility offers remarkable potential, it is essential to approach it with caution. The possible complications and long-term risks underscore the need for thorough clinical trials, rigorous regulatory oversight, and long-term patient monitoring to ensure that these treatments are both safe and effective in the long run.
Cost of Stem Cell Therapy for Infertility
12-A. Overview of Costs
Stem cell therapy for infertility represents a cutting-edge approach with the potential to address the underlying causes of reproductive issues, but it comes with a significant financial cost. The price of stem cell treatments varies widely depending on several factors, including the type of stem cells used, the complexity of the procedure, the location of the clinic, and the specific infertility issues being addressed.
Autologous stem cell therapy, where stem cells are harvested from the patient’s own body, typically costs between $5,000 and $25,000 per treatment session. This range accounts for the procedures involved in extracting, processing, and reintroducing the stem cells into the body. For instance, bone marrow-derived mesenchymal stem cells (MSCs), commonly used in these therapies, require a complex harvesting process, which can drive up the costs.
Allogeneic stem cell therapy, which involves stem cells derived from a donor, can be more expensive due to the additional steps required to ensure compatibility and reduce the risk of immune rejection. Costs for allogeneic treatments can range from $10,000 to $30,000 or more per session. This price includes the expenses related to sourcing, screening, and testing the donor cells, as well as the necessary immunosuppressive therapies that might be required.
Induced pluripotent stem cell (iPSC) therapy is one of the most costly forms of stem cell treatment due to the advanced techniques involved in reprogramming adult cells back into a pluripotent state. The cost of iPSC treatments can easily exceed $50,000 per session, reflecting the complexity of the process and the cutting-edge nature of the technology.
Additionally, the total cost of stem cell therapy for infertility often includes pre-treatment consultations, diagnostic tests, post-treatment monitoring, and follow-up care, which can add several thousand dollars to the overall expense. It’s also important to note that multiple treatment sessions may be necessary to achieve the desired results, further increasing the total cost.
Moreover, stem cell therapy is still considered experimental for many infertility conditions, which means that insurance coverage is typically limited or non-existent. Patients often have to bear the full cost out of pocket, which can be a significant financial burden, especially considering the uncertainty of the outcomes.
12-B. Comparison with Traditional Treatments
When compared to traditional infertility treatments, the cost of stem cell therapy is generally higher. However, the potential benefits and long-term effectiveness of stem cell therapy could make it a more cost-effective solution for certain patients, particularly those who have not had success with conventional methods.
In vitro fertilization (IVF), one of the most common traditional treatments for infertility, typically costs between $10,000 and $15,000 per cycle in the United States. This price can vary depending on the clinic, the specific protocols used, and whether additional procedures like intracytoplasmic sperm injection (ICSI) or preimplantation genetic testing (PGT) are required. While IVF has a moderate success rate, particularly for younger women, the costs can quickly add up if multiple cycles are needed, which is often the case.
Hormonal treatments and ovulation induction therapies, such as those involving Clomiphene Citrate (Clomid) or gonadotropins, are less expensive, typically ranging from a few hundred to a few thousand dollars per cycle. However, these treatments are often used in conjunction with other fertility treatments, which can increase the overall cost. Furthermore, the effectiveness of hormonal treatments diminishes with age and in cases of more complex infertility issues, which can necessitate more invasive and expensive treatments like IVF.
Surgical interventions for infertility, such as laparoscopic surgery for endometriosis or varicocelectomy for male infertility, can cost anywhere from $5,000 to $15,000 depending on the complexity of the procedure and the surgeon’s expertise. While surgery can be effective in resolving certain structural issues, it does not guarantee a successful pregnancy and may need to be combined with other fertility treatments.
In comparison, stem cell therapy offers the potential to address the root causes of infertility by regenerating damaged tissues and improving the overall reproductive environment, which could reduce the need for repeated cycles of IVF or other treatments. For example, a woman with diminished ovarian reserve may spend tens of thousands of dollars on multiple IVF cycles with no guarantee of success, whereas stem cell therapy might restore her ovarian function, improving her chances of conceiving naturally or with fewer IVF attempts.
However, the higher upfront cost of stem cell therapy, combined with its experimental status, means that it is often viewed as a last resort after other treatments have failed. Patients must weigh the cost-benefit ratio of stem cell therapy against the potential success rates of more established treatments. For those who have exhausted traditional options without success, the investment in stem cell therapy might be justified by the possibility of achieving pregnancy after years of failed attempts.
Ultimately, while stem cell therapy for infertility is more expensive than traditional treatments, its ability to potentially offer a lasting solution rather than a temporary fix could make it a worthwhile investment for certain patients. As research progresses and the technology becomes more widely available, costs may decrease, making stem cell therapy a more accessible option for a broader range of patients.
Recent Advances in Stem Cell Research for Infertility
13-A. Latest Studies and Innovations
Stem cell research has rapidly evolved, offering new hope for treating infertility by addressing some of its most challenging underlying causes. Recent studies and innovations have expanded our understanding of how stem cells can be used to regenerate reproductive tissues, enhance gamete quality, and improve overall fertility outcomes.
One of the most significant advances is the development of induced pluripotent stem cells (iPSCs), which are adult cells reprogrammed to return to a pluripotent state, similar to embryonic stem cells. iPSCs can differentiate into any cell type, including those necessary for reproduction, such as oocytes and sperm. Recent studies have demonstrated that iPSCs can be used to generate functional gametes in vitro. For example, researchers have successfully created oocytes from iPSCs derived from somatic cells, paving the way for new treatments for women with diminished ovarian reserves or premature ovarian failure. These lab-generated oocytes have shown the potential to mature and be fertilized, offering a groundbreaking approach to overcoming female infertility.
Similarly, iPSCs have been used to generate spermatogonial stem cells (SSCs), which can then differentiate into sperm. This innovation is particularly promising for men with non-obstructive azoospermia, where no sperm is produced due to the failure of spermatogenesis. Studies have shown that these lab-generated sperm can be used in assisted reproductive technologies (ART) like in vitro fertilization (IVF), potentially restoring fertility in men who were previously considered infertile.
Another exciting area of research involves the use of mesenchymal stem cells (MSCs) to rejuvenate aging ovarian tissue and improve egg quality. Recent clinical trials have explored the use of MSCs derived from bone marrow or adipose tissue, which are injected directly into the ovaries. Early results suggest that MSCs can enhance the ovarian environment, promote follicle development, and improve hormone production, leading to increased fertility in women with poor ovarian reserve. This approach is particularly valuable for older women or those who have undergone treatments like chemotherapy, which can damage ovarian function.
Endometrial stem cells have also gained attention for their role in treating conditions like Asherman’s syndrome and chronic endometritis, which can severely impact uterine receptivity and fertility. Recent studies have focused on using these stem cells to regenerate the endometrial lining, improving the chances of successful embryo implantation and reducing the risk of miscarriage. Clinical trials have shown promising results, with women receiving endometrial stem cell therapy experiencing improved uterine lining thickness and higher pregnancy rates.
13-B. Emerging Technologies
The integration of cutting-edge technologies such as CRISPR and 3D bioprinting with stem cell research is further pushing the boundaries of infertility treatment, offering new possibilities for personalized and effective therapies.
CRISPR-Cas9, a revolutionary gene-editing technology, has been instrumental in advancing stem cell research for infertility. CRISPR allows for precise modifications of the genome, enabling researchers to correct genetic defects that contribute to infertility. For example, CRISPR has been used to repair mutations in genes responsible for reproductive disorders, such as those affecting spermatogenesis or oocyte maturation. By editing these genes in stem cells, researchers can create healthy gametes free from genetic abnormalities that might otherwise impair fertility or lead to hereditary diseases. This approach holds promise for treating genetic causes of infertility and for improving the success rates of ART by ensuring that the gametes used are genetically sound.
Moreover, CRISPR is being explored for its potential to enhance the differentiation of stem cells into specific reproductive cell types, making it easier to generate high-quality oocytes and sperm in the lab. This technology could lead to more efficient and reliable production of gametes for use in fertility treatments, potentially overcoming some of the limitations currently faced in generating functional reproductive cells from stem cells.
3D bioprinting is another emerging technology that is revolutionizing the field of regenerative medicine, including infertility treatment. 3D bioprinting involves creating complex tissue structures by layering living cells and biomaterials. In the context of infertility, researchers are exploring the use of 3D bioprinting to create functional ovarian and testicular tissues. These bioprinted structures could potentially be transplanted into patients to restore normal reproductive function.
For instance, 3D bioprinting has been used to create ovarian tissue scaffolds that can support the survival and function of oocytes. These scaffolds mimic the natural architecture of the ovary, providing a supportive environment for follicle development and hormone production. Early research suggests that these bioprinted tissues could be used to restore fertility in women with damaged or absent ovaries, offering a new avenue for treating infertility that does not rely on donor eggs.
Similarly, 3D bioprinting is being explored for the creation of testicular tissue capable of producing sperm. By using stem cells to populate these bioprinted scaffolds, researchers aim to develop functional testicular tissue that can be implanted into men with non-obstructive azoospermia or other conditions affecting sperm production. This approach could potentially provide a long-term solution for male infertility by restoring the body’s ability to produce healthy sperm naturally.
The combination of stem cell research with CRISPR and 3D bioprinting represents a powerful synergy that could lead to unprecedented advances in infertility treatment. These technologies not only enhance the potential of stem cell therapies but also open new frontiers in personalized medicine, where treatments can be tailored to the specific genetic and biological needs of individual patients. As these technologies continue to evolve, they are likely to play a central role in the future of reproductive medicine, offering new hope to those struggling with infertility.
The Future of Infertility Treatment with Stem Cells
The future of infertility treatment with stem cells is not only about broad advancements but also about personalization—tailoring therapies to the unique needs of individual patients. Stem cell therapy is ideally suited to personalized medicine, as it can be adapted to address the specific biological and genetic factors that contribute to a patient’s infertility.
One of the most promising aspects of personalized stem cell therapy is the potential to use autologous stem cells—cells derived from the patient’s own body. These cells are less likely to be rejected by the immune system and can be engineered to address the specific causes of infertility in each patient. For example, a woman with a genetic condition that affects egg quality could have her stem cells genetically edited using CRISPR to correct the defect before the cells are used to generate healthy oocytes. Similarly, a man with non-obstructive azoospermia could receive stem cell therapy designed to regenerate sperm production based on his unique genetic and physiological profile. Personalized stem cell therapy could also be used to optimize the uterine environment for embryo implantation. By analyzing the specific characteristics of a woman’s endometrial lining, doctors could develop a stem cell treatment plan that enhances uterine receptivity, reduces inflammation, and promotes a successful pregnancy. This approach could be particularly beneficial for women who have experienced recurrent implantation failure or miscarriages. In addition to improving treatment outcomes, personalized stem cell therapies could also reduce the physical and emotional burden associated with infertility treatment. Traditional fertility treatments often involve a trial-and-error approach, where patients undergo multiple cycles of IVF or other interventions before achieving success. Personalized stem cell therapy could streamline this process by providing more targeted and effective treatments from the outset, reducing the need for multiple cycles and increasing the likelihood of success with the first attempt.
The future of infertility treatment with stem cells also holds the promise of combining therapies to achieve the best possible outcomes. For example, stem cell therapy could be used in conjunction with hormonal treatments, surgery, or ART to create a comprehensive treatment plan tailored to the patient’s specific needs. This holistic approach could address multiple factors contributing to infertility simultaneously, offering a more effective and efficient path to conception.
As personalized stem cell therapies become more refined, they are likely to play a central role in the future of reproductive medicine. By tailoring treatments to the unique needs of each patient, these therapies have the potential to improve success rates, reduce the physical and emotional toll of infertility, and offer new hope to individuals and couples facing the challenges of infertility.
Conclusion
The potential of stem cell therapy in treating infertility is immense, and we are only beginning to scratch the surface of what these therapies can achieve. While there are still challenges to overcome, particularly in terms of ensuring safety, efficacy, and accessibility, the advances made so far are promising.
As stem cell research continues to evolve, these therapies will likely become an integral part of reproductive medicine, offering solutions where traditional treatments have failed. The ability to regenerate reproductive tissues, improve the quality of gametes, and enhance the overall fertility environment represents a paradigm shift in how infertility is treated. In the future, stem cell-based treatments could become the standard of care for many forms of infertility, providing personalized, effective, and minimally invasive options for patients. This would not only improve the chances of conception but also reduce the physical and emotional toll that infertility can take on individuals and couples.
In conclusion, stem cell therapy holds the promise of transforming the landscape of infertility treatment, offering new hope to those who have long struggled with the challenges of starting a family. As research progresses, the dream of overcoming infertility through the power of stem cells may soon become a reality for many.
FAQs
- What are the success rates of stem cell therapy in treating infertility?
The success rates of stem cell therapy for infertility are still being determined, as the treatment is relatively new and largely experimental. Initial studies and clinical trials have shown promising results, particularly in improving egg and sperm quality, regenerating ovarian tissue, and enhancing uterine receptivity. However, these success rates vary depending on factors such as the underlying cause of infertility, the type of stem cells used, and the patient’s overall health. As research progresses and more data becomes available, a clearer picture of the success rates will emerge.
- Who is eligible for stem cell-based infertility treatments?
Eligibility for stem cell-based infertility treatments typically depends on the underlying cause of infertility and the patient’s medical history. Candidates may include individuals with diminished ovarian reserve, premature ovarian failure, non-obstructive azoospermia, or uterine factor infertility. Patients who have not had success with conventional infertility treatments such as IVF may also be considered for stem cell therapy. However, because this therapy is still experimental, it is generally offered as part of clinical trials or in specialized medical centers. Eligibility criteria are determined by the specifics of each trial or treatment program.
- Are there any risks associated with this treatment?
Yes, there are risks associated with stem cell therapy for infertility, as with any medical treatment. Possible complications include immune reactions, infections, and the risk of tumor formation if the stem cells proliferate uncontrollably. There is also the potential for incomplete differentiation of stem cells, leading to ineffective treatment or unexpected side effects. Long-term risks are still being studied, and patients undergoing stem cell therapy are often monitored closely to ensure safety. It is important to discuss these risks with a healthcare provider to make an informed decision.
- How does stem cell therapy compare to other infertility treatments?
Stem cell therapy offers a novel approach to infertility treatment by addressing underlying issues such as poor egg or sperm quality, damaged reproductive tissues, or uterine receptivity. Unlike traditional treatments like IVF or hormonal therapies, which often focus on managing symptoms or temporarily enhancing fertility, stem cell therapy aims to regenerate and repair reproductive tissues at a fundamental level. While it shows great promise, stem cell therapy is generally more expensive and less widely available than other treatments, and it is still considered experimental. However, for patients who have not had success with conventional treatments, stem cell therapy could offer a more long-term and potentially effective solution.
- How long does it take to see results?
The timeline for seeing results from stem cell therapy for infertility can vary depending on the type of treatment and the individual patient’s condition. In some cases, improvements in reproductive function may be observed within a few months, particularly if the therapy is focused on enhancing egg or sperm quality. For more complex treatments, such as those involving tissue regeneration, it may take longer—several months to a year—before significant results are seen. Patients should discuss expected timelines with their healthcare provider, as follow-up care and monitoring are often required to assess the effectiveness of the treatment.
- What are the costs involved?
The costs of stem cell therapy for infertility can vary widely depending on several factors, including the type of stem cells used, the complexity of the procedure, the location of the clinic, and the specific infertility issues being treated. Generally, the costs can range from $5,000 to $50,000 or more per treatment session.
- Autologous stem cell therapy typically costs between $5,000 and $25,000 per session. This includes the extraction, processing, and reintroduction of the stem cells.
- Allogeneic stem cell therapy can be more expensive, with costs ranging from $10,000 to $30,000 or more due to additional steps like donor screening and immune compatibility testing.
- iPSC therapy, which involves reprogramming adult cells into a pluripotent state, is the most expensive, often exceeding $50,000 per session.
It’s important to note that these costs typically cover only the treatment itself and may not include additional expenses such as consultations, diagnostic tests, post-treatment monitoring, and follow-up care. Since stem cell therapy for infertility is still considered experimental in many cases, insurance coverage is usually limited, meaning patients often need to pay out of pocket.
Patients should also consider the possibility of needing multiple treatment sessions to achieve the desired results, which can further increase the overall cost.