Content table
Introduction to Chemotherapy and Fertility
Chemotherapy, a cornerstone of modern cancer treatment, involves the use of powerful drugs to target and destroy rapidly dividing cancer cells. While highly effective in treating various types of cancer, chemotherapy is not without its side effects. Among the most significant of these is the potential impact on fertility—a concern that is often overlooked in the urgency to treat cancer but is critically important for many patients.
Fertility, the ability to conceive and bear offspring, is a vital aspect of life for many individuals and couples. It is intricately linked to personal identity, future plans, and overall quality of life. For cancer patients, the prospect of losing fertility can add an additional layer of emotional and psychological stress to an already overwhelming experience. This concern is particularly acute for younger patients and those who have not yet started or completed their families.
Chemotherapy’s impact on fertility arises from its mechanism of action. The drugs used in chemotherapy are designed to kill rapidly dividing cells, a characteristic of cancer cells. However, this mechanism is not selective and can also affect other rapidly dividing cells in the body, including those in the reproductive system. This unintended collateral damage can lead to a range of fertility issues, from temporary disruptions in reproductive function to permanent infertility. The extent to which chemotherapy affects fertility depends on several factors, including the type of cancer being treated, the specific chemotherapy drugs used, the dosage, and the patient’s age and gender. Women and men experience these effects differently, with varying implications for their reproductive health. For women, chemotherapy can damage the ovaries, leading to a reduction in the number of viable eggs, hormonal imbalances, and in some cases, premature ovarian failure (POF). Men, on the other hand, may experience a decrease in sperm count, reduced sperm quality, and potential long-term damage to sperm-producing cells. Despite these risks, there are ways to mitigate the impact of chemotherapy on fertility. Advances in medical science have led to the development of various fertility preservation techniques, allowing many cancer patients to retain the possibility of having biological children in the future. These techniques range from well-established methods like egg and sperm freezing to more experimental approaches such as ovarian and testicular tissue preservation. However, the decision to pursue fertility preservation is a deeply personal one, influenced by a range of factors including the type and stage of cancer, the urgency of treatment, personal and cultural beliefs, and financial considerations. It requires careful consultation with healthcare providers, including oncologists and fertility specialists, to ensure that patients are fully informed of their options and the potential risks involved.
In summary, understanding the impact of chemotherapy on fertility is crucial for cancer patients, particularly those of reproductive age. By being informed and proactive, patients can take steps to preserve their fertility and maintain the possibility of starting or growing their families after cancer treatment. This introduction sets the stage for a more detailed exploration of how chemotherapy affects fertility, the factors that influence these effects, and the options available for fertility preservation.

Mechanism of Chemotherapy
Chemotherapy is a fundamental aspect of cancer treatment that works by targeting and destroying cancer cells. To understand how chemotherapy can impact fertility, it’s essential to first grasp its underlying mechanisms and the nature of the drugs involved. Chemotherapy operates primarily by attacking cells that divide rapidly, a characteristic feature of cancer cells. Under normal conditions, cells in the body grow, divide, and die in a controlled manner. However, cancer cells lose this regulatory mechanism and multiply uncontrollably, forming tumors and spreading to other parts of the body. Chemotherapy drugs disrupt this process by interfering with the DNA replication or the cell division process, ultimately leading to the death of the cancer cells.
2-A. Types of Chemotherapy Drugs
Chemotherapy is not a one-size-fits-all treatment; there are various types of chemotherapy drugs, each with a different mechanism of action and potential side effects. These drugs can be broadly classified into several categories based on their mode of action:
- Alkylating Agents
Alkylating agents, such as cyclophosphamide, work by adding an alkyl group to the DNA of cancer cells. This modification prevents the DNA from properly replicating, leading to cell death. While effective against many types of cancer, alkylating agents are also known to cause significant damage to the reproductive organs, especially the ovaries, and testes, leading to a high risk of infertility. - Antimetabolites
Antimetabolites, including drugs like methotrexate and 5-fluorouracil, mimic the building blocks of DNA or RNA, tricking the cancer cells into incorporating these substances during replication. This interference results in the production of faulty DNA, which triggers cell death. Antimetabolites tend to affect rapidly dividing cells, not only in tumors but also in healthy tissues like the bone marrow, gastrointestinal tract, and reproductive organs. - Mitotic Inhibitors
Drugs such as paclitaxel and vincristine fall under this category. They work by inhibiting mitosis, the process by which cells divide. By disrupting the microtubules essential for cell division, mitotic inhibitors prevent cancer cells from multiplying. However, because these drugs target all rapidly dividing cells, they can also impact spermatogenesis (sperm production) and oogenesis (egg production). - Topoisomerase Inhibitors
Topoisomerase inhibitors, like doxorubicin, interfere with the enzyme topoisomerase, which is necessary for DNA replication and repair. These drugs prevent the unwinding of DNA, a crucial step in cell division, leading to cell death. Their use is associated with a range of side effects, including potential impacts on fertility, as they can damage the DNA of reproductive cells. - Cytotoxic Antibiotics
Unlike traditional antibiotics, which fight infections, cytotoxic antibiotics, such as bleomycin and daunorubicin, kill cancer cells by binding to DNA and inhibiting both DNA and RNA synthesis. These drugs are effective against various cancers but are also associated with significant risks to reproductive health due to their broad impact on cellular functions.
2-B. General Side Effects of Chemotherapy
While chemotherapy is effective in combating cancer, its non-selective nature means that it can also harm healthy cells, particularly those that divide rapidly. This includes not only cancer cells but also cells in the bone marrow, digestive tract, hair follicles, and reproductive organs. The damage to these healthy cells results in the common side effects of chemotherapy, which can be both short-term and long-term.
- Hematologic Side Effects
Since chemotherapy affects the bone marrow, where blood cells are produced, patients often experience anemia, increased risk of infection due to low white blood cell counts, and easy bruising or bleeding from a reduced platelet count. - Gastrointestinal Side Effects
The cells lining the gastrointestinal tract are also rapidly dividing, making them susceptible to chemotherapy. This can lead to nausea, vomiting, diarrhea, and mouth sores, which are some of the most well-known side effects of chemotherapy. - Hair Loss (Alopecia)
Hair follicles, another group of rapidly dividing cells, are often damaged during chemotherapy, resulting in hair loss. Although this side effect is usually temporary, it can be distressing for patients. - Reproductive Side Effects
Chemotherapy can significantly impact reproductive organs, leading to potential infertility. In women, chemotherapy can reduce the ovarian reserve, leading to early menopause or even complete loss of ovarian function. In men, chemotherapy can reduce sperm count, affect sperm quality, and in some cases, lead to permanent infertility.
2-C. Fertility-Specific Side Effects
The reproductive system is particularly vulnerable to chemotherapy due to its reliance on rapidly dividing cells. In women, the ovaries contain a finite number of eggs, and chemotherapy can accelerate the loss of these eggs, potentially leading to premature ovarian failure. This not only impacts a woman’s ability to conceive but can also trigger early menopause, with associated symptoms such as hot flashes, night sweats, and osteoporosis. In men, the testes produce sperm continuously, but this process can be disrupted by chemotherapy. Some chemotherapy drugs are more likely to cause temporary or permanent azoospermia, a condition where no sperm are produced. Even if sperm production resumes after treatment, the quality of sperm—such as motility and DNA integrity—may be compromised, affecting fertility and the likelihood of a successful pregnancy.
In conclusion, the way chemotherapy works at the cellular level is both its strength and its Achilles’ heel. While it is effective in destroying cancer cells, its lack of specificity means that other rapidly dividing cells, including those in the reproductive system, are also at risk. This dual effect is what makes chemotherapy a powerful but potentially fertility-damaging treatment, necessitating careful consideration and, where possible, proactive steps to preserve fertility before beginning treatment.
The Biological Impact of Chemotherapy on Fertility
Chemotherapy’s primary goal is to eradicate cancer cells, but its aggressive approach can also have profound effects on the body’s reproductive system. The impact on fertility is a significant concern for many patients, as chemotherapy can damage the reproductive organs and impair the production of gametes—sperm in men and eggs in women. Understanding the biological mechanisms through which chemotherapy affects fertility can help patients make informed decisions about their treatment and explore options for fertility preservation.
3-A. Impact on Ovaries
The ovaries, which house a woman’s eggs, are particularly vulnerable to the effects of chemotherapy. A woman is born with a finite number of eggs, and unlike sperm, which are produced continuously, the number of eggs in the ovaries decreases over time. Chemotherapy drugs can accelerate this natural decline, leading to a reduction in ovarian reserve—a measure of the number and quality of eggs remaining in the ovaries.
Chemotherapy-induced damage to the ovaries can occur through several mechanisms:
- Direct Follicular Damage
The follicles within the ovaries contain immature eggs, and chemotherapy can directly destroy these follicles, reducing the number of eggs available for ovulation. Some chemotherapy drugs, especially alkylating agents like cyclophosphamide, are known to be highly gonadotoxic (toxic to reproductive organs), causing significant follicular depletion. - Vascular Damage
Chemotherapy can also impair blood flow to the ovaries by damaging the blood vessels that supply them. This reduced blood supply can lead to hypoxia (lack of oxygen) and subsequent follicular damage, further diminishing ovarian reserve. - Hormonal Disruption
The ovaries are not only responsible for producing eggs but also for secreting hormones like estrogen and progesterone, which regulate the menstrual cycle. Chemotherapy can disrupt this hormonal balance, leading to irregular periods or amenorrhea (the absence of menstruation). This hormonal imbalance is often an early sign of reduced ovarian function and can precede the onset of menopause. - Premature Ovarian Failure (POF)
In severe cases, the cumulative damage from chemotherapy can lead to premature ovarian failure, where the ovaries stop functioning entirely before the age of 40. POF not only results in infertility but also triggers early menopause, with symptoms such as hot flashes, mood swings, and increased risk of osteoporosis due to low estrogen levels.
3-B. Impact on Testes
The testes, responsible for producing sperm and testosterone, are also at significant risk during chemotherapy. The process of spermatogenesis (the production of sperm) is continuous and involves the rapid division of cells—a process that chemotherapy targets.
Chemotherapy’s impact on the testes includes:
- Spermatogenesis Disruption
The cells involved in spermatogenesis are highly susceptible to chemotherapy drugs, particularly those that target rapidly dividing cells. This can lead to a reduction in sperm count (oligospermia) or, in severe cases, complete cessation of sperm production (azoospermia). The extent of this disruption depends on the type and dosage of chemotherapy drugs used. - DNA Damage in Sperm
Even if sperm production continues during or after chemotherapy, the genetic material within the sperm may be damaged. Chemotherapy can cause breaks in the DNA strands of sperm, leading to compromised sperm quality. This damage can result in reduced fertility, increased risk of miscarriage, or genetic abnormalities in offspring. - Testosterone Production
While chemotherapy primarily affects spermatogenesis, it can also impact testosterone production in some cases. Lower testosterone levels can lead to symptoms such as reduced libido, fatigue, and loss of muscle mass. However, significant reductions in testosterone are less common compared to the effects on sperm production. - Long-Term Fertility Risks
For some men, sperm production may recover after the completion of chemotherapy, but this recovery can take months or even years. In others, particularly those who have undergone high-dose chemotherapy or treatment with highly gonadotoxic drugs, fertility may be permanently impaired. The long-term impact on fertility is also influenced by age, with older men being less likely to recover normal sperm production after treatment.
3-C. Effects on Gamete Production (Sperm and Eggs)
Chemotherapy’s effects on gamete production—whether eggs in women or sperm in men—are a direct consequence of its action on rapidly dividing cells. Both eggs and sperm are produced through a series of cell divisions, which are disrupted by chemotherapy.
- In Women
The production of viable eggs is dependent on the health and number of ovarian follicles. Chemotherapy can reduce the number of these follicles, resulting in fewer eggs being available for fertilization. The remaining eggs may also be of lower quality, with a higher likelihood of chromosomal abnormalities. This reduction in both quantity and quality of eggs can significantly decrease a woman’s chances of conceiving naturally after chemotherapy. - In Men
Sperm production is a continuous process, but it is highly sensitive to chemotherapy. The damage to the germ cells that produce sperm can lead to a significant drop in sperm count. Additionally, the surviving sperm may carry damaged DNA, which can affect fertility outcomes. While some men may regain normal sperm production over time, the recovery is not guaranteed, and the quality of sperm may remain compromised.
3-D. Differential Impact Based on Chemotherapy Drug Type
Not all chemotherapy drugs have the same level of impact on fertility. Alkylating agents, for example, are known to be particularly damaging to reproductive cells, with a high likelihood of causing infertility. Other drugs, like certain antimetabolites, may have a lower risk but can still cause significant harm depending on the dosage and duration of treatment.
3-E. Gender Differences in Fertility Impact
The biological differences between male and female reproductive systems mean that chemotherapy affects them in different ways. Women are born with a finite number of eggs, making them particularly vulnerable to treatments that deplete ovarian follicles. Men, on the other hand, continuously produce sperm, but the process is highly sensitive to chemotherapy, and the recovery of sperm production can be unpredictable.
In conclusion, the biological impact of chemotherapy on fertility is profound and multifaceted. Both the ovaries in women and the testes in men are vulnerable to damage from chemotherapy, leading to potential infertility. Understanding these effects is crucial for patients and healthcare providers alike, as it underscores the importance of discussing fertility preservation options before starting treatment.

Factors Influencing the Impact on Fertility
The impact of chemotherapy on fertility is not uniform across all patients; it varies widely depending on several key factors. Understanding these factors is crucial for assessing the risk of infertility and making informed decisions about fertility preservation before starting treatment. This section explores how the type of cancer, the patient’s age, the specific chemotherapy drugs used, and the duration of treatment influence the extent to which fertility may be affected.
4-A. Type of Cancer
The type of cancer being treated can significantly influence the impact of chemotherapy on fertility. Certain cancers directly involve reproductive organs, while others may require aggressive chemotherapy that indirectly affects fertility.
- Reproductive System Cancers
Cancers that originate in or involve the reproductive organs, such as ovarian, testicular, and cervical cancers, often require treatments that are particularly harsh on fertility. In these cases, surgery, radiation, and chemotherapy may all combine to increase the risk of infertility. For instance, ovarian cancer treatment often involves the removal of one or both ovaries, in addition to chemotherapy, leading to a direct loss of fertility. - Systemic Cancers
Cancers like leukemia, lymphoma, and breast cancer may not originate in the reproductive organs but often require high-dose chemotherapy that can affect fertility. For example, women with breast cancer who undergo chemotherapy, especially with drugs like cyclophosphamide, are at risk of ovarian damage, leading to reduced fertility or early menopause. - Pediatric Cancers
Children and adolescents undergoing chemotherapy for cancers such as Hodgkin’s lymphoma or osteosarcoma are particularly vulnerable to long-term fertility issues. Because their reproductive systems are still developing, the impact of chemotherapy can be profound and may not become apparent until they reach adulthood and attempt to conceive.
4-B. Patient’s Age
Age is one of the most critical factors in determining how chemotherapy will impact fertility. The reproductive system’s ability to recover from chemotherapy is closely linked to the patient’s age at the time of treatment.
- Women
In women, age is directly related to ovarian reserve—the number and quality of eggs remaining in the ovaries. Younger women typically have a higher ovarian reserve, which can provide some buffer against the damaging effects of chemotherapy. However, as women age, their ovarian reserve naturally declines, making them more susceptible to chemotherapy-induced infertility. Women over the age of 35 are particularly at risk because their ovarian reserve is already diminished, and the likelihood of chemotherapy causing permanent ovarian failure is higher. - Men
While men produce sperm continuously throughout their lives, sperm quality and quantity generally decline with age. Younger men are more likely to recover sperm production after chemotherapy, although the extent of recovery can still vary. Older men may experience a more significant impact, with a reduced likelihood of sperm production returning to pre-treatment levels. Additionally, older age in men may exacerbate the effects of chemotherapy on sperm DNA, potentially leading to fertility challenges even if sperm production resumes.
4-C. Specific Chemotherapy Drugs Used
Not all chemotherapy drugs carry the same risk of infertility. Some are more gonadotoxic—meaning they are more likely to damage the reproductive organs and affect fertility.
- Alkylating Agents
Alkylating agents, such as cyclophosphamide, busulfan, and ifosfamide, are among the most harmful to reproductive cells. These drugs work by cross-linking DNA strands, preventing cell division and leading to cell death. This mechanism is highly effective against cancer cells but is also particularly damaging to the cells in the ovaries and testes. As a result, patients treated with alkylating agents face a high risk of infertility. - Platinum Compounds
Drugs like cisplatin and carboplatin, commonly used in treating testicular, ovarian, and lung cancers, can also significantly impact fertility. These drugs cause DNA cross-linking similar to alkylating agents and can damage the reproductive cells in both men and women. - Antimetabolites
While less gonadotoxic than alkylating agents, antimetabolites like methotrexate and 5-fluorouracil can still affect fertility, particularly when used in high doses or for extended periods. These drugs interfere with DNA and RNA synthesis, which can disrupt spermatogenesis and oogenesis, the processes by which sperm and eggs are produced. - Anthracyclines and Taxanes
These classes of drugs, including doxorubicin and paclitaxel, are commonly used in breast cancer treatment. They are less likely to cause infertility compared to alkylating agents but can still pose a risk, especially when used in combination with other chemotherapy drugs. - Cumulative Drug Effect
Often, cancer treatment involves a combination of different chemotherapy drugs, which can have a cumulative effect on fertility. The more gonadotoxic drugs included in the treatment regimen, the higher the likelihood of infertility. Additionally, repeated cycles of chemotherapy increase the risk, as the damage to reproductive organs can accumulate over time.
4-D. Duration and Dosage of Treatment
The length of chemotherapy treatment and the dosage administered are also crucial factors in determining the risk of infertility.
- High-Dose Chemotherapy
High-dose chemotherapy, often used in preparation for bone marrow or stem cell transplants, poses a significant risk to fertility. The intense nature of the treatment means that it targets not only cancer cells but also rapidly dividing healthy cells, including those in the reproductive system. Patients undergoing high-dose chemotherapy are at a much higher risk of permanent infertility. - Extended Treatment Duration
Chemotherapy regimens that extend over a long period can lead to cumulative damage to the ovaries or testes. Each cycle of chemotherapy increases the likelihood of reproductive harm, particularly if the treatment involves drugs that are known to be gonadotoxic. For example, patients undergoing multiple cycles of alkylating agents over several months may face a significantly higher risk of infertility compared to those on shorter, less intense regimens. - Interval Between Treatment Cycles:
The time between chemotherapy cycles can also influence fertility outcomes. Short intervals between treatments do not allow sufficient time for the reproductive organs to recover, leading to greater cumulative damage. On the other hand, longer intervals might give the body a chance to repair some of the damage, potentially reducing the impact on fertility.
4-E. Other Considerations
Beyond the primary factors mentioned, several additional considerations can influence how chemotherapy impacts fertility:
- Pre-Existing Health Conditions
Patients with pre-existing conditions, such as polycystic ovary syndrome (PCOS) in women or low sperm count in men, may be more susceptible to chemotherapy-induced infertility. These conditions can exacerbate the effects of chemotherapy on reproductive health. - Lifestyle Factors
Lifestyle factors such as smoking, excessive alcohol consumption, and poor diet can also impact fertility. When combined with the stress of chemotherapy, these factors can further reduce the chances of maintaining fertility after treatment. - Genetic Predispositions
Some individuals may have a genetic predisposition to infertility, which can be triggered or exacerbated by chemotherapy. For example, women with a family history of early menopause may be more likely to experience premature ovarian failure after chemotherapy.
In conclusion, the impact of chemotherapy on fertility is influenced by a complex interplay of factors including the type of cancer, the patient’s age, the specific chemotherapy drugs used, and the duration and intensity of the treatment. Understanding these factors is essential for assessing individual risk and exploring fertility preservation options before beginning chemotherapy.
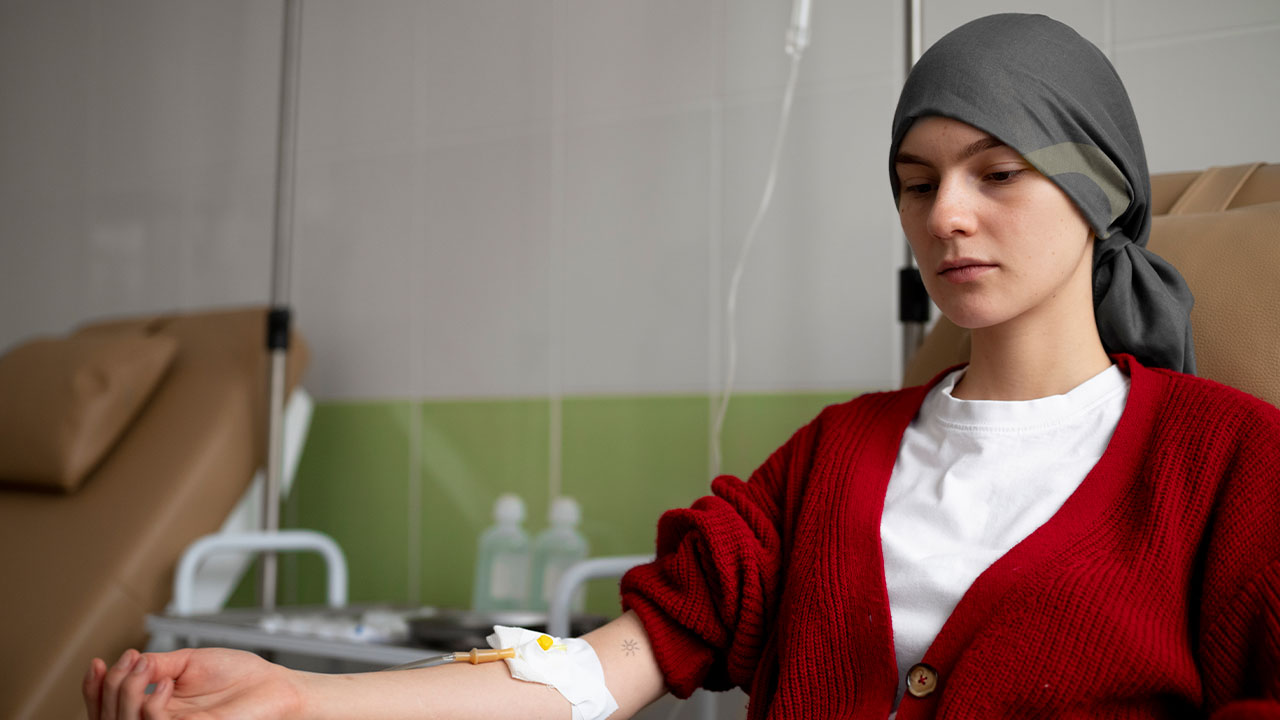
Chemotherapy-Induced Infertility in Women
Chemotherapy-induced infertility is a significant concern for women undergoing cancer treatment, particularly those of reproductive age. The effects of chemotherapy on female fertility are profound, primarily due to its impact on the ovaries, which are responsible for producing eggs and regulating the menstrual cycle. This section delves into how chemotherapy can lead to infertility in women, focusing on the depletion of ovarian reserve, disruptions to the menstrual cycle, and the potential for premature ovarian failure (POF).
5-A. Effects on the Ovarian Reserve
The ovarian reserve refers to the total number of viable eggs a woman has available for fertilization. Women are born with a finite number of eggs, which gradually decreases over time. By puberty, a woman has about 300,000 to 400,000 eggs, but only a small fraction of these will ever mature and be released during ovulation. Chemotherapy can significantly accelerate the depletion of this ovarian reserve, leading to a reduced number of eggs and, consequently, a diminished likelihood of conception.
- Direct Damage to Ovarian Follicles
Chemotherapy drugs, especially those classified as alkylating agents (e.g., cyclophosphamide), are known to be highly gonadotoxic. They can directly damage the ovarian follicles, which contain immature eggs. This damage is often irreversible, leading to a permanent reduction in the ovarian reserve. The extent of this damage depends on the type and dose of chemotherapy, with some drugs being more harmful than others. - Reduction in Egg Quality
In addition to reducing the number of eggs, chemotherapy can also affect the quality of the remaining eggs. Eggs damaged by chemotherapy may have chromosomal abnormalities, which can lead to difficulties in conceiving, increased risk of miscarriage, or congenital disabilities in offspring. The reduction in egg quality is particularly concerning for older women, who may already have a higher proportion of eggs with chromosomal issues due to age. - Age-Related Vulnerability
The impact of chemotherapy on ovarian reserve is also closely linked to the woman’s age at the time of treatment. Younger women typically have a larger ovarian reserve and may be more resilient to the effects of chemotherapy, although they are not immune. Older women, particularly those over the age of 35, face a higher risk of significant depletion of their ovarian reserve because they start with fewer eggs and are closer to natural menopause. For these women, chemotherapy can hasten the onset of menopause and lead to infertility.
5-B. Disruptions to the Menstrual Cycle
Chemotherapy can also disrupt the regularity of the menstrual cycle, which is a key indicator of a woman’s reproductive health. The menstrual cycle is governed by a delicate balance of hormones produced by the ovaries, including estrogen and progesterone. Chemotherapy can interfere with this hormonal balance, leading to a range of menstrual irregularities.
- Amenorrhea (Absence of Menstruation)
One of the most common side effects of chemotherapy is amenorrhea, the temporary or permanent cessation of menstruation. Amenorrhea occurs because chemotherapy can suppress ovarian function, leading to a significant drop in the production of hormones needed to maintain the menstrual cycle. In younger women, amenorrhea may be temporary, with periods resuming months or even years after treatment. However, in older women or those who receive high doses of chemotherapy, amenorrhea may become permanent, signaling the onset of menopause and the end of fertility. - Irregular Menstrual Cycles
Even if menstruation continues during chemotherapy, cycles may become irregular. Women might experience longer or shorter cycles, heavier or lighter bleeding, or spotting between periods. These irregularities can make it difficult to predict ovulation, complicating efforts to conceive naturally. Irregular cycles also indicate that the ovaries are under stress, which could be a precursor to more severe fertility issues. - Hormonal Imbalance
Chemotherapy’s impact on the ovaries can lead to a significant hormonal imbalance. Reduced estrogen levels can cause symptoms similar to those experienced during menopause, such as hot flashes, night sweats, and vaginal dryness. These symptoms are not only uncomfortable but also indicative of reduced ovarian function, which can impair fertility. Hormonal imbalances may also affect other aspects of health, including bone density, mood, and cardiovascular function, further complicating a woman’s overall well-being.
5-C. Premature Ovarian Failure (POF)
One of the most severe consequences of chemotherapy-induced ovarian damage is premature ovarian failure (POF). POF, also known as primary ovarian insufficiency, occurs when the ovaries stop functioning normally before the age of 40. This condition leads to infertility, as the ovaries no longer produce viable eggs, and is often accompanied by early menopause.
- Mechanism of POF
POF occurs when chemotherapy accelerates the natural decline of ovarian function to the point where the ovaries no longer respond to the body’s hormonal signals to produce eggs. The exact mechanism is multifactorial, involving the direct destruction of ovarian follicles, vascular damage to the ovaries, and the resultant hormonal disruptions. Once POF sets in, the chances of spontaneous conception are extremely low, and the condition is generally considered irreversible. - Risk Factors for POF
Several factors increase the risk of developing POF as a result of chemotherapy. These include the use of highly gonadotoxic drugs, high cumulative doses of chemotherapy, and older age at the time of treatment. Genetic predisposition also plays a role; women with a family history of early menopause or other reproductive issues may be more susceptible to POF. Additionally, women who receive pelvic radiation in combination with chemotherapy are at an even higher risk due to the compounded effects of both treatments on the ovaries. - Consequences of POF
The onset of POF has significant implications beyond infertility. Women with POF experience early menopause, which brings with it all the associated symptoms, such as hot flashes, night sweats, and vaginal atrophy. Early menopause also increases the risk of long-term health issues, including osteoporosis and cardiovascular disease, due to the loss of estrogen’s protective effects. Psychologically, the diagnosis of POF can be devastating, particularly for women who have not yet started or completed their families. - Management of POF
While POF itself cannot be reversed, its symptoms can be managed through hormone replacement therapy (HRT), which can help alleviate menopausal symptoms and reduce the risk of osteoporosis and cardiovascular disease. However, HRT does not restore fertility. For women with POF who wish to have children, options such as egg donation or adoption may be explored, but these come with their own set of emotional and practical challenges.
5-D. Psychosocial Impact of Chemotherapy-Induced Infertility
The prospect of infertility can be a significant emotional burden for women undergoing chemotherapy. For many, the ability to conceive and bear children is a fundamental part of their identity and future plans. The loss of this ability can lead to feelings of grief, depression, and anxiety, exacerbated by the physical and emotional stress of cancer treatment itself. Women facing chemotherapy-induced infertility may also struggle with feelings of isolation, particularly if they feel their concerns about fertility are overshadowed by the urgency of cancer treatment. Support from healthcare providers, family, and friends is crucial during this time. Counseling and support groups can provide a space for women to process their emotions and connect with others who are facing similar challenges. Additionally, early discussions about fertility preservation before starting chemotherapy can help mitigate some of the psychological impacts by giving women a sense of control over their reproductive future.
In conclusion, chemotherapy-induced infertility in women is a complex issue that involves a combination of ovarian damage, hormonal disruptions, and potential premature ovarian failure. The extent of these effects varies depending on individual factors, but for many women, the risk is significant. Understanding these risks is essential for making informed decisions about fertility preservation and managing the emotional and physical consequences of infertility.
Chemotherapy-Induced Infertility in Men
Chemotherapy-induced infertility is a significant concern for men undergoing cancer treatment, particularly those who wish to preserve their ability to father biological children. The impact of chemotherapy on male fertility primarily revolves around its effects on sperm production, sperm quality, and long-term reproductive health. This section explores these issues in detail, highlighting the biological mechanisms behind chemotherapy-induced infertility in men and the potential long-term consequences.
6-A. Impact on Sperm Production
Sperm production, or spermatogenesis, is a continuous process that occurs in the testes. This process involves the rapid division and maturation of cells to produce viable sperm, making it highly susceptible to the effects of chemotherapy, which targets rapidly dividing cells.
- Reduction in Sperm Count (Oligospermia)
Chemotherapy drugs can significantly reduce the number of sperm produced by the testes, a condition known as oligospermia. The extent of this reduction depends on the type and dosage of the chemotherapy drugs used. Some drugs, particularly alkylating agents like cyclophosphamide and cisplatin, are highly gonadotoxic and can drastically lower sperm counts. In some cases, sperm production may decrease to such a low level that it becomes difficult or impossible to conceive naturally. - Azoospermia (Complete Absence of Sperm)
In more severe cases, chemotherapy can lead to azoospermia, the complete absence of sperm in the ejaculate. Azoospermia occurs when the chemotherapy drugs cause extensive damage to the spermatogenic cells in the testes, halting sperm production altogether. This condition can be temporary or permanent, depending on the extent of the damage and the specific drugs used. High-dose chemotherapy regimens, such as those used before bone marrow or stem cell transplants, are particularly likely to cause permanent azoospermia. - Recovery of Sperm Production
The potential for sperm production to recover after chemotherapy varies widely among individuals. For some men, particularly younger patients or those treated with lower doses of less gonadotoxic drugs, sperm production may resume after a period of months or even years. However, the recovery process is unpredictable, and in many cases, sperm counts may never return to pre-treatment levels. The likelihood of recovery also decreases with age, as the regenerative capacity of the testes diminishes over time.
6-B. Impact on Sperm Quality
Even when sperm production resumes after chemotherapy, the quality of the sperm produced can be significantly affected. Sperm quality is critical for successful fertilization and healthy embryo development, and chemotherapy can impair various aspects of sperm function.
- DNA Damage
One of the most concerning effects of chemotherapy on sperm is the potential for DNA damage. Chemotherapy drugs can cause breaks in the DNA strands within sperm cells, leading to compromised genetic integrity. This damage can result in reduced fertility, as sperm with fragmented DNA are less likely to successfully fertilize an egg. Additionally, DNA-damaged sperm increase the risk of miscarriage or genetic abnormalities in offspring. - Sperm Motility and Morphology
Chemotherapy can also affect sperm motility (the ability to swim) and morphology (shape and structure). Sperm motility is essential for the sperm to travel through the female reproductive tract and reach the egg for fertilization. Reduced motility, a condition known as asthenozoospermia, can significantly impair a man’s fertility. Similarly, abnormalities in sperm morphology (teratozoospermia) can affect the sperm’s ability to penetrate and fertilize the egg, further reducing the likelihood of conception. - Long-Term Effects on Sperm Quality
The long-term effects of chemotherapy on sperm quality can persist even if sperm production resumes. Men who regain the ability to produce sperm after chemotherapy may still have a higher proportion of sperm with DNA damage or abnormal morphology, which can affect fertility outcomes. This long-term impact underscores the importance of fertility preservation measures, such as sperm banking, before starting chemotherapy.
6-C. Long-Term Reproductive Health Consequences
The impact of chemotherapy on male fertility extends beyond immediate sperm production and quality, with potential long-term consequences for reproductive health.
- Permanent Infertility
For some men, particularly those exposed to high-dose or highly gonadotoxic chemotherapy, the damage to the testes may be so severe that fertility is permanently impaired. Permanent infertility means that natural conception is no longer possible, and assisted reproductive technologies (ART) may be required to achieve pregnancy. In cases of azoospermia where no viable sperm are produced, options such as sperm donation or adoption may need to be considered. - Hypogonadism
Chemotherapy can also affect the testes’ ability to produce testosterone, leading to a condition known as hypogonadism. Testosterone is crucial for maintaining male reproductive health, as well as other physiological functions such as muscle mass, bone density, and libido. Symptoms of hypogonadism include fatigue, decreased libido, erectile dysfunction, and reduced muscle mass. In severe cases, hormone replacement therapy (HRT) may be necessary to manage these symptoms, although it does not restore fertility. - Psychosocial Impact
The prospect of infertility can be a significant psychological burden for men undergoing chemotherapy. Concerns about the loss of fertility can exacerbate the emotional stress of cancer treatment, leading to feelings of anxiety, depression, and diminished self-esteem. For many men, the ability to father biological children is closely tied to their sense of identity and future plans, and the potential loss of this ability can be deeply distressing. Psychological support, including counseling and support groups, can play a vital role in helping men cope with these challenges.
6-D. Fertility Preservation Options
Given the significant risk of chemotherapy-induced infertility, fertility preservation is an important consideration for men before starting cancer treatment. Sperm banking is the most common and effective method of preserving fertility. This process involves collecting and freezing sperm samples before chemotherapy begins. The frozen sperm can then be used for assisted reproductive techniques such as intrauterine insemination (IUI) or in vitro fertilization (IVF) in the future. For boys who have not yet reached puberty and cannot produce sperm, experimental techniques such as testicular tissue freezing may offer potential, although these methods are still under research.
In conclusion, chemotherapy-induced infertility in men is a complex issue that affects both sperm production and quality, with long-term implications for reproductive health. Understanding these risks and exploring fertility preservation options before beginning chemotherapy is crucial for men who wish to maintain the possibility of fathering biological children in the future.
Preserving Fertility Before Chemotherapy
Preserving fertility before starting chemotherapy is a critical consideration for cancer patients who hope to have biological children in the future. The aggressive nature of chemotherapy, which targets rapidly dividing cells, can severely impact reproductive organs, leading to temporary or permanent infertility. Fortunately, there are several advanced techniques available for both men and women to preserve their fertility before undergoing chemotherapy. This section outlines these options and discusses their respective success rates, providing valuable information for patients to make informed decisions.
7-A. Fertility Preservation in Women
Women face unique challenges when it comes to fertility preservation because of the finite number of eggs they have and the sensitivity of the ovaries to chemotherapy. The following methods are commonly used to preserve fertility in women:
- Egg Freezing (Oocyte Cryopreservation)
Egg freezing is one of the most well-established and widely used methods for fertility preservation in women. The process involves stimulating the ovaries with hormones to produce multiple eggs, which are then retrieved and frozen for future use. These frozen eggs can later be thawed, fertilized with sperm in a laboratory setting, and implanted in the uterus through in vitro fertilization (IVF).
The success of egg freezing depends on the woman’s age at the time of freezing and the number of eggs retrieved. Younger women tend to have higher success rates because their eggs are generally of better quality. Studies indicate that women under 35 who freeze a sufficient number of eggs (typically around 15-20) have a good chance of achieving pregnancy in the future. The success rate of live births from frozen eggs ranges from 20% to 50%, depending on the quality and number of eggs and the woman’s age when the eggs were frozen.
- Embryo Freezing (Embryo Cryopreservation)
Similar to egg freezing, embryo freezing involves the stimulation of the ovaries to produce eggs. However, in this method, the eggs are fertilized with sperm before being frozen as embryos. This option is typically chosen by women who have a partner or are willing to use donor sperm at the time of preservation.
Success Rates: Embryo freezing generally offers higher success rates compared to egg freezing because embryos, once fertilized, are more resilient to the freezing and thawing process. The success rate of achieving a live birth from frozen embryos can range from 30% to 60%, depending on the woman’s age at the time of freezing and the quality of the embryos.
- Ovarian Tissue Freezing (Ovarian Tissue Cryopreservation)
Ovarian tissue freezing is an experimental method that involves surgically removing and freezing a portion of ovarian tissue containing immature eggs. After chemotherapy, this tissue can be re-implanted into the woman’s body, where it may restore hormonal function and potentially allow for natural conception.
Success Rates: Ovarian tissue freezing is still considered experimental, and success rates are not as well-established as those for egg and embryo freezing. However, several successful pregnancies have been reported using this method, particularly in younger women. The procedure is increasingly being used for prepubescent girls who do not yet produce mature eggs and therefore cannot undergo egg or embryo freezing.
- Ovarian Suppression with GnRH Agonists
This method involves administering gonadotropin-releasing hormone (GnRH) agonists to suppress ovarian function temporarily during chemotherapy. The theory is that by putting the ovaries into a “resting” state, they may be less vulnerable to the damaging effects of chemotherapy.
Success Rates: The effectiveness of ovarian suppression in preserving fertility is still debated. Some studies suggest a protective effect, particularly in reducing the risk of premature ovarian failure (POF), while others show no significant benefit. Given the uncertainty, ovarian suppression is often used in combination with other fertility preservation methods rather than as a standalone option.
- In Vitro Maturation (IVM)
In cases where there is not enough time to undergo the full process of ovarian stimulation and egg retrieval, in vitro maturation (IVM) may be an option. IVM involves retrieving immature eggs from the ovaries, maturing them in a laboratory setting, and then freezing them.
Success Rates: IVM is less commonly used and is considered more experimental than traditional egg or embryo freezing. Success rates are generally lower due to the challenges involved in maturing eggs outside the body. However, for patients with time constraints or certain medical conditions, IVM offers an alternative that can be paired with egg or embryo freezing.
7-B. Fertility Preservation in Men
Men have more straightforward and established options for preserving fertility before chemotherapy, largely due to the continuous nature of sperm production. The following methods are commonly used:
- Sperm Banking (Sperm Cryopreservation)
Sperm banking is the most common and reliable method of fertility preservation for men. The process involves collecting and freezing sperm samples before starting chemotherapy. These samples can be stored for many years and used for future assisted reproductive technologies (ART) such as intrauterine insemination (IUI) or in vitro fertilization (IVF).
Success Rates: Sperm freezing is highly effective, with success rates largely dependent on the quality of the sperm before freezing and the ART method used for conception. Generally, sperm retains its viability well during the freezing and thawing process, and pregnancies have been successfully achieved with sperm stored for decades.
- Testicular Sperm Extraction (TESE)
For men who are unable to produce a sperm sample through ejaculation due to blockage or absence of sperm in the ejaculate, testicular sperm extraction (TESE) may be an option. TESE involves surgically retrieving sperm directly from the testicular tissue, which can then be frozen for future use.
Success Rates: The success of TESE depends on the underlying cause of the man’s infertility and the presence of viable sperm in the testicular tissue. TESE is often paired with IVF and intracytoplasmic sperm injection (ICSI) to maximize the chances of fertilization.
- Testicular Tissue Freezing
For prepubescent boys who have not yet begun producing sperm, testicular tissue freezing is an experimental option. This involves freezing a small portion of testicular tissue with the hope that, in the future, the tissue can be re-implanted to restore fertility or used to mature sperm in vitro.
Success Rates: Testicular tissue freezing is still in the experimental stages, and no pregnancies have yet been reported using this method. However, research is ongoing, and the technique holds promise for preserving fertility in boys who would otherwise have no other options.
- Hormonal Treatments
Some experimental approaches involve using hormonal treatments to protect sperm production during chemotherapy. However, these methods are still under investigation, and their effectiveness is not yet proven.
Success Rates: Due to the experimental nature of hormonal treatments for fertility preservation in men, success rates are not well-established, and these methods are generally not recommended as a primary preservation strategy.
7-C. Considerations and Decision-Making
Choosing the best fertility preservation method involves considering various factors, including the patient’s age, type of cancer, urgency of starting treatment, personal preferences, and financial considerations. Fertility preservation is time-sensitive, as some methods, like egg or embryo freezing, require several weeks to complete. Therefore, patients need to consult with both their oncologist and a fertility specialist as early as possible after a cancer diagnosis to discuss their options.
In conclusion, preserving fertility before chemotherapy is a critical step for patients who wish to maintain the possibility of having biological children in the future. With advancements in reproductive technology, both men and women have several effective options to safeguard their fertility, allowing them to focus on cancer treatment with greater peace of mind about their reproductive futures.
Fertility Preservation Techniques in Detail
Fertility preservation is a critical consideration for cancer patients who wish to retain the option of having biological children after treatment. Advances in reproductive medicine have made it possible to preserve fertility through various techniques, each with its own set of procedures, benefits, and success rates. This section provides an in-depth exploration of these fertility preservation techniques, focusing on egg and sperm freezing, as well as newer methods like ovarian and testicular tissue preservation.
8-A. Egg Freezing (Oocyte Cryopreservation)
Egg freezing is one of the most established fertility preservation techniques available for women. It involves harvesting and freezing a woman’s eggs before they are exposed to the potentially harmful effects of chemotherapy.
- Process
- Ovarian Stimulation: The process begins with ovarian stimulation, where the patient is administered hormonal medications to stimulate the ovaries to produce multiple eggs. This phase typically lasts 10 to 14 days and involves daily injections of hormones such as follicle-stimulating hormone (FSH) and luteinizing hormone (LH).
- Egg Retrieval: Once the eggs have matured, they are retrieved from the ovaries through a minor surgical procedure called transvaginal ultrasound aspiration. This procedure is usually performed under light sedation and involves the insertion of a needle through the vaginal wall to reach the ovaries.
- Cryopreservation: The retrieved eggs are then assessed for quality, and the viable ones are frozen using a technique called vitrification, which involves rapidly cooling the eggs to prevent the formation of ice crystals that could damage the cells.
- Benefits
- Preservation of Reproductive Potential: Egg freezing allows women to preserve their reproductive potential at a time when their eggs are most viable, typically before the effects of chemotherapy can cause significant damage.
- Flexibility: Frozen eggs can be stored for many years, providing women with flexibility regarding when or if they choose to use them.
- Challenges
- Time Sensitivity: The process of ovarian stimulation and egg retrieval takes several weeks, which may not be feasible for women who need to begin chemotherapy immediately.
- Success Rates: The success of egg freezing depends on the woman’s age at the time of freezing and the number of eggs retrieved. Generally, younger women have higher success rates, with a live birth rate per frozen egg ranging from 2% to 12%.
- Ideal Candidates
- Women who are of reproductive age and wish to delay childbearing due to cancer treatment.
- Women who have sufficient time before starting chemotherapy to undergo ovarian stimulation and egg retrieval.
8-B. Sperm Freezing (Sperm Cryopreservation)
Sperm freezing is the most common and straightforward method for preserving fertility in men. It involves collecting and freezing sperm samples before chemotherapy begins.
- Process
- Sperm Collection: The process starts with the collection of sperm through masturbation. In cases where ejaculation is not possible due to medical conditions or other factors, sperm may be collected through testicular sperm extraction (TESE), which involves retrieving sperm directly from the testes.
- Analysis and Freezing: The sperm sample is analyzed for motility, concentration, and morphology. Viable sperm are then frozen using a method called slow freezing or vitrification. The sperm can be stored in liquid nitrogen at extremely low temperatures for many years.
- Benefits
- High Success Rates: Sperm freezing is a highly effective method of fertility preservation. Sperm can remain viable for decades, with successful pregnancies reported from sperm frozen for over 20 years.
- Quick and Non-Invasive: The process is relatively simple, non-invasive, and can be completed quickly, making it ideal for men who need to start chemotherapy urgently.
- Challenges
- Quantity and Quality: Men with low sperm counts or poor sperm quality may face challenges in obtaining a sufficient quantity of viable sperm for freezing. In such cases, multiple samples may need to be collected.
- Ideal Candidates
- Men who wish to preserve their fertility before undergoing cancer treatment that could affect sperm production.
- Boys who have reached puberty and are able to produce a sperm sample.
8-C. Embryo Freezing (Embryo Cryopreservation)
Embryo freezing involves fertilizing a woman’s eggs with sperm before freezing the resulting embryos for future use. This method is typically chosen by women who have a partner or are willing to use donor sperm.
- Process
- Ovarian Stimulation and Egg Retrieval: The initial steps are the same as those for egg freezing, involving ovarian stimulation and egg retrieval.
- Fertilization: The retrieved eggs are fertilized with sperm in a laboratory setting, using either traditional in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI), where a single sperm is injected directly into the egg.
- Embryo Development: The fertilized eggs develop into embryos over several days. The most viable embryos are then selected for freezing using vitrification.
- Benefits
- Higher Success Rates: Embryo freezing generally offers higher success rates than egg freezing because embryos, once fertilized, are more resilient to the freezing and thawing process. The success rate of live births from frozen embryos ranges from 30% to 60%, depending on the woman’s age at the time of freezing and the quality of the embryos.
- Established Method: Embryo freezing is a well-established and widely used method in assisted reproductive technology.
- Challenges
- Need for a Partner or Donor Sperm: This method requires either a male partner or donor sperm, which may not be suitable for all women.
- Ethical and Legal Considerations: There may be ethical and legal considerations regarding the use of frozen embryos, particularly if the couple’s relationship status changes or in the case of the death of one partner.
- Ideal Candidates
- Women who have a partner or are willing to use donor sperm and who have sufficient time before starting chemotherapy to undergo the necessary procedures.
8-D. Ovarian Tissue Freezing (Ovarian Tissue Cryopreservation)
Ovarian tissue freezing is an experimental method that involves removing and freezing ovarian tissue containing immature eggs. This tissue can later be re-implanted after chemotherapy, potentially restoring ovarian function and fertility.
- Process
- Tissue Removal: The procedure begins with a laparoscopic surgery to remove a portion of the ovary. The ovarian tissue, which contains immature eggs, is then processed and frozen.
- Re-implantation: After the patient completes chemotherapy and is in remission, the frozen ovarian tissue can be thawed and re-implanted into the patient’s body. In some cases, the tissue is re-implanted near the remaining ovary; in others, it may be placed in a different location in the body.
- Benefits
- Restoration of Natural Fertility: One of the key benefits of ovarian tissue freezing is the potential to restore natural fertility. Once re-implanted, the tissue can begin producing hormones and releasing eggs, allowing for natural conception.
- No Need for Ovarian Stimulation: Unlike egg or embryo freezing, ovarian tissue freezing does not require hormonal stimulation, making it an option for women who cannot undergo ovarian stimulation or who need to start chemotherapy immediately.
- Challenges
- Experimental Nature: Although ovarian tissue freezing has resulted in successful pregnancies, it is still considered experimental, and the success rates are lower and less well-documented compared to egg and embryo freezing.
- Surgical Risks: The procedure requires surgery both to remove and to re-implant the tissue, which carries risks such as infection and complications from anesthesia.
- Ideal Candidates
- Women who cannot delay chemotherapy for ovarian stimulation.
- Prepubescent girls who do not yet produce mature eggs and therefore cannot undergo egg or embryo freezing.
8-E. Testicular Tissue Freezing
Testicular tissue freezing is an emerging fertility preservation technique primarily used for prepubescent boys who are not yet producing sperm. This method involves freezing a small portion of testicular tissue, with the hope that future advances will allow the tissue to be used to restore fertility.
- Process
- Tissue Collection: The procedure begins with a biopsy of the testicular tissue. The collected tissue, which contains spermatogonial stem cells (precursors to sperm), is then frozen for future use.
- Future Use: Research is ongoing to develop methods for using frozen testicular tissue to generate mature sperm or to re-implant the tissue to restore natural sperm production.
- Benefits
- Hope for Future Fertility: For boys who would otherwise have no options for fertility preservation, testicular tissue freezing offers the possibility of future fertility once the technology is fully developed.
- Non-Invasive Collection for Adults: In cases where sperm cannot be retrieved through ejaculation, testicular tissue freezing offers an alternative method for preserving fertility.
- Challenges
- Experimental Status: Testicular tissue freezing is still highly experimental, with no successful pregnancies reported to date using this method. The technique’s effectiveness will depend on future advancements in reproductive technology.
- Surgical Risks: The tissue collection procedure is surgical, which may carry risks, particularly in young children.
- Ideal Candidates
- Prepubescent boys who cannot produce sperm but wish to preserve their future fertility options.
- Adult men who cannot ejaculate and wish to preserve fertility through tissue freezing.
8-F. In Vitro Maturation (IVM)
In vitro maturation (IVM) is a fertility preservation technique that involves retrieving immature eggs from the ovaries and maturing them in the laboratory before freezing.
- Process
- Egg Retrieval: Immature eggs are retrieved from the ovaries, typically without the need for the extensive hormonal stimulation required for conventional egg retrieval.
- Laboratory Maturation: The immature eggs are then matured in the laboratory environment over the course of a few days.
- Freezing: Once matured, the eggs are frozen using vitrification.
- Benefits
- Reduced Hormonal Stimulation: IVM requires little to no hormonal stimulation, making it a suitable option for women who cannot undergo or wish to avoid the side effects of hormone treatments.
- Speed: The process is quicker than traditional egg freezing, which is beneficial for women who need to begin chemotherapy promptly.
- Challenges
- Lower Success Rates: The success rates of IVM are generally lower than those of conventional egg or embryo freezing. The procedure is less common and considered more experimental.
- Specialized Equipment and Expertise: IVM requires specific laboratory conditions and expertise, which may not be available at all fertility centers.
- Ideal Candidates
- Women who cannot delay chemotherapy but still wish to preserve their fertility.
- Women with medical conditions that contraindicate hormonal stimulation.
In conclusion, fertility preservation techniques have evolved significantly, offering cancer patients a variety of options to safeguard their reproductive futures. Whether through egg or sperm freezing, tissue preservation, or newer experimental methods, these techniques provide hope for patients who wish to start or expand their families after cancer treatment. Each method has its own set of benefits, challenges, and success rates, and the choice of technique should be tailored to the individual’s medical condition, treatment timeline, and personal preferences.
Fertility Preservation and Cancer Type
Cancer treatment presents unique challenges to fertility preservation, which can vary significantly depending on the type of cancer and the specific therapies used. The location of the cancer, the urgency of treatment, and the potential impact of both the cancer and its treatment on reproductive organs all influence the approach to fertility preservation. This section explores how different types of cancer affect fertility preservation strategies, highlighting the specific challenges and solutions associated with each type.
9-A. Breast Cancer
Breast cancer is one of the most common cancers affecting women of reproductive age, and its treatment often poses significant risks to fertility.
- Impact of Treatment
- Chemotherapy: Many breast cancer treatment regimens include chemotherapy drugs like cyclophosphamide, which are known to be highly gonadotoxic and can lead to premature ovarian failure (POF). This risk is particularly high in women over the age of 35, whose ovarian reserve is already diminished.
- Hormonal Therapy: Hormonal treatments, such as tamoxifen, are commonly used in hormone receptor-positive breast cancer. These treatments are usually administered over several years and can delay childbearing until the woman’s fertility has naturally declined.
- Surgery and Radiation: While surgery and radiation typically do not directly affect fertility, radiation therapy to areas near the reproductive organs (such as the chest) can sometimes impact ovarian function, especially if scatter radiation affects nearby tissues.
- Fertility Preservation Strategies
- Egg or Embryo Freezing: For women with breast cancer, egg or embryo freezing is the most common method of fertility preservation. Because hormonal stimulation can temporarily raise estrogen levels—potentially risky for hormone-sensitive cancers—special protocols that minimize estrogen exposure, such as letrozole or tamoxifen co-treatment, are often used.
- Ovarian Suppression: The use of GnRH agonists to temporarily suppress ovarian function during chemotherapy is another option, although its effectiveness as a fertility preservation strategy remains debated.
- Timing Considerations: The timing of fertility preservation is critical. Ideally, fertility preservation procedures should be completed before chemotherapy or hormonal therapy begins. However, for women with aggressive cancers requiring immediate treatment, the window for these procedures may be very narrow.
9-B. Lymphoma and Leukemia
Lymphomas (such as Hodgkin’s lymphoma and non-Hodgkin’s lymphoma) and leukemias often affect younger individuals, including adolescents and young adults who may be particularly concerned about their future fertility.
- Impact of Treatment
- Chemotherapy: Treatment for lymphomas and leukemias frequently involves aggressive chemotherapy regimens that can severely impair fertility. Alkylating agents, such as cyclophosphamide, are a key component of these regimens and are known to cause significant damage to the ovaries and testes.
- Stem Cell Transplantation: High-dose chemotherapy followed by stem cell transplantation, a common treatment for certain leukemias and lymphomas, poses an even greater risk to fertility. This treatment can lead to permanent infertility due to the high doses of gonadotoxic agents used.
- Fertility Preservation Strategies
- Sperm Banking: For male patients, sperm banking is a standard and highly effective method of preserving fertility. Because sperm production can be severely affected by chemotherapy, it is crucial to bank sperm before treatment begins.
- Egg or Embryo Freezing: Female patients are advised to undergo egg or embryo freezing before starting chemotherapy. Given the urgency of treatment for many lymphomas and leukemias, fertility preservation procedures must be completed quickly, often within a few weeks of diagnosis.
- Ovarian Tissue Freezing: For patients who cannot delay treatment or who are prepubescent, ovarian tissue freezing may be an option. This experimental technique offers the possibility of restoring fertility in the future, although it is still in the early stages of clinical use.
9-C. Testicular Cancer
Testicular cancer primarily affects young men, typically between the ages of 15 and 35, a demographic for whom fertility is often a major concern.
- Impact of Treatment
- Surgery: Orchiectomy, the surgical removal of the affected testicle, is the standard treatment for testicular cancer. While removal of one testicle does not necessarily lead to infertility, it can reduce overall sperm production, particularly if the remaining testicle is affected by subclinical conditions.
- Chemotherapy: Chemotherapy is often used after surgery to eliminate any remaining cancer cells, particularly in cases of metastatic disease. The drugs used in chemotherapy, such as cisplatin, can impair sperm production, leading to temporary or permanent infertility.
- Radiation Therapy: Radiation therapy, though less commonly used for testicular cancer, can also affect fertility, particularly if the remaining testicle is exposed to radiation.
- Fertility Preservation Strategies
- Sperm Banking: Sperm banking is strongly recommended for all men diagnosed with testicular cancer, ideally before surgery or chemotherapy begins. This is the most reliable method for preserving fertility, as it ensures that sperm is available for future use, regardless of the treatment’s impact on fertility.
- Testicular Tissue Freezing: For prepubescent boys who are not yet producing sperm, testicular tissue freezing may be considered. While still experimental, this technique offers hope for future fertility preservation.
9-D. Gynecological Cancers
Gynecological cancers, including ovarian, cervical, and endometrial cancers, often involve direct treatment of the reproductive organs, posing a significant challenge to fertility preservation.
- Impact of Treatment
- Surgery: Surgical treatment for gynecological cancers often involves the removal of reproductive organs, such as the ovaries or uterus, leading to immediate and irreversible infertility.
- Chemotherapy and Radiation: Chemotherapy and radiation therapy can also affect fertility by damaging the ovaries or the uterine lining. Radiation, particularly when directed at the pelvis, can cause significant harm to the ovaries and may lead to premature ovarian failure.
- Fertility Preservation Strategies
- Egg or Embryo Freezing: For women with early-stage ovarian or cervical cancer, egg or embryo freezing before surgery or chemotherapy may be an option if the ovaries are still functional. In some cases, ovarian transposition (moving the ovaries out of the radiation field) can be performed to protect ovarian function.
- Ovarian Tissue Freezing: Ovarian tissue freezing is an option for women who cannot delay cancer treatment or who have cancers that involve the ovaries. This method is also considered for younger patients or those who wish to preserve their fertility without undergoing hormonal stimulation.
- Uterine Preservation: In cases of early-stage cervical or endometrial cancer, fertility-sparing surgery that preserves the uterus may be possible. This approach is usually combined with other fertility preservation methods, such as egg or embryo freezing, to maximize the chances of future pregnancy.
9-E. Pediatric Cancers
Children diagnosed with cancer face unique challenges when it comes to fertility preservation, particularly because many have not yet reached reproductive maturity.
- Impact of Treatment
- Chemotherapy: Pediatric cancer treatments often involve high doses of chemotherapy, which can severely affect future fertility. Prepubescent boys and girls are at risk of losing their ability to produce sperm or eggs due to the gonadotoxic effects of these treatments.
- Radiation Therapy: Radiation, particularly to the pelvic area, can damage reproductive organs and impair future fertility in children.
- Fertility Preservation Strategies
- Ovarian and Testicular Tissue Freezing: For prepubescent children who are not yet capable of producing mature sperm or eggs, ovarian and testicular tissue freezing are the primary options for fertility preservation. These methods are still experimental but offer the potential for future fertility restoration.
- Sperm Banking: For adolescent boys who have reached puberty, sperm banking is a straightforward and effective option. It is important to collect and freeze sperm before starting cancer treatment.
9-F. Bone and Soft Tissue Sarcomas
Bone and soft tissue sarcomas often affect adolescents and young adults, making fertility preservation an important consideration.
- Impact of Treatment
- Chemotherapy: The treatment of sarcomas often involves aggressive chemotherapy regimens, including drugs like ifosfamide and doxorubicin, which can cause significant damage to the reproductive organs.
- Radiation Therapy: Radiation therapy, particularly when directed at the pelvis or abdomen, can impair fertility by damaging the ovaries, testes, or uterine lining.
- Fertility Preservation Strategies
- Egg or Embryo Freezing: For women diagnosed with sarcomas, egg or embryo freezing is the most common method of fertility preservation. The timing of these procedures must be carefully coordinated with the start of cancer treatment.
- Sperm Banking: Sperm banking is recommended for male patients before beginning chemotherapy or radiation therapy. For boys who have not yet reached puberty, testicular tissue freezing may be considered.
In conclusion, the type of cancer and its treatment significantly influence fertility preservation strategies. Each type of cancer presents unique challenges that require tailored approaches to ensure that patients have the best possible options for preserving their fertility. Early consultation with fertility specialists and oncologists is essential to develop a personalized fertility preservation plan that takes into account the specific needs and circumstances of each patient.
Post-Chemotherapy Fertility
Chemotherapy, while effective in treating cancer, often poses significant risks to fertility. However, many patients are concerned about their ability to conceive after completing treatment. The journey to parenthood following chemotherapy is fraught with both possibilities and challenges, as the impact of treatment on fertility can vary widely depending on factors such as the type and dose of chemotherapy, the patient’s age, and pre-treatment fertility. This section explores the potential for fertility recovery after chemotherapy, the timeline for the return of fertility, and the available options for those who face difficulties conceiving naturally.
10-A. Possibilities of Conceiving After Chemotherapy
The possibility of conceiving after chemotherapy depends largely on the extent of the damage to the reproductive organs and the individual’s overall reproductive health. While some patients may regain their fertility naturally, others may face significant challenges.
- Natural Fertility Recovery
- In Women: After chemotherapy, some women may experience a return of normal ovarian function, including regular menstrual cycles and the ability to conceive naturally. The likelihood of this recovery depends on several factors, including the woman’s age at the time of treatment, the specific chemotherapy drugs used, and the baseline ovarian reserve before treatment. Younger women, particularly those under 35, are more likely to experience a return of fertility, as their ovarian reserve tends to be higher. In some cases, menstrual cycles may resume within months, but it can sometimes take years for normal ovarian function to return fully.
- In Men: Men may also experience a return to normal sperm production after chemotherapy, although this can take time. The duration of azoospermia (absence of sperm) varies, with some men regaining sperm production within a few months to a year after treatment, while others may take several years or may never fully recover. The ability to conceive naturally depends on the recovery of both sperm count and sperm quality, which can be significantly affected by the type and dose of chemotherapy received.
- Permanent Infertility
For some patients, chemotherapy may cause permanent damage to the reproductive organs, leading to infertility. This is particularly common in patients who have undergone high-dose chemotherapy or who have been treated with highly gonadotoxic drugs such as alkylating agents. Women may experience premature ovarian failure (POF), where the ovaries stop functioning entirely, leading to early menopause and a loss of fertility. In men, permanent azoospermia may occur, making natural conception impossible without assisted reproductive technologies (ART).
10-B. Timeline for Fertility to Return
The timeline for fertility to return after chemotherapy is highly individualized and can vary significantly between patients.
- Women:
- Menstrual Cycle Resumption: The return of regular menstrual cycles is often the first sign that fertility may be returning in women. This can occur as early as a few months after completing chemotherapy, but for some women, it may take several years. The return of menstruation does not guarantee full fertility, as the quality and quantity of eggs may still be compromised.
- Monitoring Ovarian Function: Women who wish to conceive after chemotherapy should have their ovarian function monitored regularly. This can include blood tests to measure hormone levels (such as FSH and AMH) and ultrasounds to assess the antral follicle count (AFC). These tests can help determine the likelihood of natural conception and inform decisions about the timing of trying to conceive.
- Men:
- Sperm Recovery: In men, the recovery of sperm production typically takes longer than the return of menstrual cycles in women. Sperm production may resume within a year after chemotherapy, but it can take up to five years or more for some men to see a significant improvement in sperm count and quality. Regular semen analyses can help track progress and determine when it might be possible to attempt natural conception.
- Patience and Monitoring: Like women, men should undergo regular monitoring, including semen analysis, to assess sperm count, motility, and morphology. The presence of healthy, motile sperm is a positive sign that natural conception may be possible.
10-C. Options if Natural Conception is Not Possible
For patients who do not regain their fertility naturally after chemotherapy, there are several options available to help them achieve parenthood.
- Assisted Reproductive Technologies (ART)
- In Vitro Fertilization (IVF): IVF is a common option for couples facing infertility after chemotherapy. In cases where women have a reduced ovarian reserve but are still ovulating, IVF can be used to retrieve the eggs and fertilize them in a laboratory setting. For men with low sperm counts or poor sperm quality, IVF combined with intracytoplasmic sperm injection (ICSI) can be used to improve the chances of fertilization. The success rates of IVF vary depending on the age of the woman and the quality of the eggs and sperm but generally range from 20% to 40% per cycle.
- Use of Donor Gametes: In cases where patients are unable to produce viable eggs or sperm after chemotherapy, the use of donor eggs, sperm, or embryos is an option. This approach allows couples to achieve pregnancy even when one or both partners are infertile. The success rates for IVF using donor eggs or sperm are generally higher than using the patient’s own gametes, particularly when the donor is younger and healthier.
- Gestational Surrogacy: For women who are unable to carry a pregnancy due to the effects of chemotherapy on the uterus or overall health, gestational surrogacy is an option. This involves implanting an embryo (created using the patient’s eggs and sperm or donor gametes) into a surrogate’s uterus. The surrogate carries the pregnancy to term, and the child is genetically related to the intended parents if their gametes are used.
- Adoption
- Adoption is another option for cancer survivors who are unable to conceive naturally or through ART. Many individuals and couples find fulfillment in building their families through adoption, whether domestic or international. While the process of adoption can be lengthy and complex, it provides a viable path to parenthood for those facing infertility after chemotherapy.
- Fertility Preservation Prior to Chemotherapy
- Use of Frozen Gametes: For patients who had the foresight to preserve their fertility before starting chemotherapy, using frozen eggs, sperm, or embryos can provide the opportunity to conceive after treatment. These preserved gametes or embryos can be used in IVF procedures to achieve pregnancy when natural conception is not possible.
- Ovarian and Testicular Tissue Transplantation: For patients who preserved ovarian or testicular tissue before chemotherapy, re-implantation of this tissue offers another potential avenue for restoring fertility. Although still considered experimental, these procedures have resulted in successful pregnancies in some cases, offering hope to patients who would otherwise have limited options.
10-D. Emotional and Psychological Considerations
The process of trying to conceive after chemotherapy can be emotionally challenging, especially for those who face infertility. The uncertainty of fertility recovery, the potential need for ART, and the possibility of permanent infertility can cause significant stress and anxiety. Support from healthcare providers, fertility specialists, counselors, and support groups is crucial for helping patients navigate these challenges.
- Coping with Uncertainty
- Patients should be prepared for the possibility that fertility may not return immediately and that achieving pregnancy may require time, patience, and possibly multiple attempts with ART. Understanding the realities of post-chemotherapy fertility and setting realistic expectations can help manage stress and disappointment.
- Seeking Support
- Counseling and support groups can provide valuable emotional support for patients dealing with fertility issues after chemotherapy. Connecting with others who have gone through similar experiences can help reduce feelings of isolation and provide practical advice and encouragement.
In conclusion, post-chemotherapy fertility is a complex and deeply personal issue that requires careful consideration and planning. While some patients may regain their fertility naturally, others may need to explore ART or alternative paths to parenthood. Regardless of the outcome, it is essential for patients to have access to comprehensive medical and emotional support as they navigate their journey toward building a family after cancer treatment.
Psychological Impacts of Chemotherapy-Induced Infertility
Chemotherapy, while essential for treating cancer, can have profound and lasting effects on fertility. For many patients, the realization that cancer treatment may compromise or eliminate their ability to have biological children is devastating. The emotional and psychological toll of chemotherapy-induced infertility is often significant, affecting not just the patient but also their relationships, self-esteem, and overall quality of life. This section explores the deep psychological impacts of infertility caused by chemotherapy and underscores the importance of counseling and support groups in helping patients navigate these challenges.
11-A. Emotional and Psychological Toll of Infertility
Infertility can trigger a complex array of emotions, including grief, loss, anger, and anxiety. For cancer patients, these feelings are often compounded by the physical and emotional strain of the cancer diagnosis and treatment itself. The following are some of the most common psychological impacts associated with chemotherapy-induced infertility:
- Grief and Loss
- Mourning the Loss of Future Parenthood: For many patients, the inability to have biological children is a profound loss. This can lead to a grieving process similar to that experienced after the death of a loved one. Patients may grieve not only the loss of their reproductive potential but also the loss of a future they had envisioned for themselves, including the possibility of raising a family.
- Impact on Identity: Parenthood is often a significant part of personal identity. When chemotherapy robs patients of the ability to conceive, it can lead to a crisis of identity, where patients struggle with feelings of inadequacy, failure, or diminished self-worth. This is particularly true for individuals who have always dreamed of becoming parents or for those whose cultural or religious values place a strong emphasis on family and childbearing.
- Anxiety and Depression
- Fear of the Unknown: The uncertainty surrounding post-chemotherapy fertility can lead to significant anxiety. Patients may worry about whether their fertility will return, the potential challenges of assisted reproductive technologies (ART), and the long-term implications of their infertility. This anxiety can be overwhelming and may persist long after cancer treatment has ended.
- Depression: The combination of cancer treatment and infertility can lead to clinical depression in some patients. The feelings of hopelessness and helplessness associated with infertility can be exacerbated by the physical and emotional toll of cancer treatment. Depression can manifest as persistent sadness, loss of interest in activities, fatigue, and difficulty concentrating, further diminishing the patient’s quality of life.
- Relationship Strain
- Impact on Romantic Relationships: Infertility can place a significant strain on romantic relationships, particularly if one partner’s fertility is affected more than the other’s. Couples may struggle with feelings of guilt, blame, or resentment, especially if they had planned to start or expand their family. The stress of infertility, combined with the demands of cancer treatment, can lead to communication breakdowns and emotional distancing.
- Challenges with Intimacy: The physical and emotional changes caused by chemotherapy, such as changes in body image, hormonal fluctuations, and sexual dysfunction, can also affect intimacy between partners. These challenges can exacerbate the emotional distance and strain caused by infertility, making it even more difficult for couples to maintain a strong, supportive relationship during and after cancer treatment.
- Social Isolation
- Feeling Different or Alone: Infertility can make patients feel isolated from their peers, particularly if they are surrounded by friends or family members who are having children. The sense of being different or “left out” can lead to withdrawal from social situations and an increased sense of loneliness.
- Stigma and Misunderstanding: Infertility is often misunderstood or stigmatized, which can make it difficult for patients to talk openly about their experiences. This can further isolate them from their support networks, as they may feel that others cannot relate to or understand their pain.
11-B. The Importance of Counseling and Support Groups
Given the significant emotional and psychological challenges associated with chemotherapy-induced infertility, access to counseling and support groups is crucial for helping patients cope with their feelings and find a sense of peace and acceptance. These resources offer a safe space for patients to explore their emotions, connect with others who are experiencing similar challenges, and develop strategies for managing the psychological impacts of infertility.
- Counseling
- Individual Therapy: One-on-one counseling with a therapist who specializes in oncology or reproductive health can be immensely beneficial. Therapy provides a private space for patients to process their emotions, work through feelings of grief and loss, and develop coping mechanisms. Cognitive-behavioral therapy (CBT), in particular, has been shown to be effective in reducing anxiety and depression in cancer patients dealing with infertility.
- Couples Therapy: For patients in relationships, couples therapy can help address the strain that infertility places on their partnership. A trained therapist can facilitate open communication between partners, helping them to express their feelings, understand each other’s perspectives, and work together to find solutions or alternative paths to parenthood. Couples therapy can also help partners strengthen their emotional connection and support each other through the challenges of infertility and cancer recovery.
- Grief Counseling: For patients struggling with the profound sense of loss associated with infertility, grief counseling can be particularly helpful. This type of therapy focuses on helping individuals come to terms with their loss, process their grief in a healthy way, and find new ways to envision their future.
- Support Groups
- Peer Support Groups: Joining a support group for individuals facing cancer-related infertility can provide a sense of community and understanding. These groups allow patients to share their experiences, exchange advice, and offer mutual support. Knowing that they are not alone in their struggles can be incredibly comforting for patients, helping to reduce feelings of isolation and loneliness.
- Online Support Communities: For those who may not have access to in-person support groups, online communities offer a valuable alternative. These forums and social media groups provide a platform for patients to connect with others facing similar challenges, regardless of geographic location. Online support communities can be particularly helpful for sharing information, finding resources, and receiving emotional support at any time.
- Workshops and Educational Programs: Many cancer centers and fertility clinics offer workshops and educational programs focused on fertility preservation, coping with infertility, and exploring alternative paths to parenthood. These programs provide patients with the knowledge and tools they need to make informed decisions about their reproductive futures and to navigate the emotional challenges of infertility.
- Holistic Approaches to Emotional Well-Being
- Mindfulness and Meditation: Mindfulness practices, such as meditation and yoga, can help patients manage the stress and anxiety associated with infertility. These practices promote relaxation, reduce emotional distress, and improve overall mental health. Mindfulness can also help patients stay present at the moment, reducing the tendency to dwell on worries about the future.
- Creative Therapies: Art therapy, music therapy, and other forms of creative expression can provide patients with a healthy outlet for their emotions. These therapies can help patients process complex feelings, such as grief and anger, in a non-verbal way, offering relief and a sense of control over their emotional well-being.
- Physical Activity: Regular physical activity has been shown to improve mood, reduce anxiety, and boost self-esteem. For patients dealing with infertility, exercise can provide a constructive way to manage stress and improve overall health, contributing to a more positive outlook on life.
The psychological impact of chemotherapy-induced infertility is profound, affecting every aspect of a patient’s life. However, with the right support, patients can navigate these challenges and find a way to move forward. Counseling, support groups, and holistic approaches to emotional well-being are essential resources for helping patients cope with the emotional and psychological toll of infertility. By addressing these issues with compassion and care, healthcare providers can help patients reclaim their sense of self, find peace with their circumstances, and explore new possibilities for their future.
Consultations and Questions to Ask Before Chemotherapy
The decision to undergo chemotherapy is often made with a sense of urgency, given the seriousness of cancer. However, for patients who wish to preserve their fertility, it is crucial to take the time to consult with fertility specialists before beginning treatment. Early consultation can help patients understand the potential impact of chemotherapy on their fertility and explore available options for preservation. This section emphasizes the importance of these consultations and provides a list of critical questions that patients should ask their doctors to make informed decisions about their reproductive future.
12-A. Importance of Consulting with Fertility Specialists
Before starting chemotherapy, it is essential for patients to understand how the treatment may affect their fertility and what steps can be taken to preserve it. Consulting with a fertility specialist offers several key benefits:
- Personalized Fertility Preservation Plan
- Every cancer patient’s situation is unique, and fertility preservation strategies must be tailored to the individual’s age, type of cancer, treatment plan, and personal desires regarding future parenthood. A fertility specialist can provide a personalized assessment and recommend the most appropriate preservation options based on the patient’s specific circumstances.
- Timing and Coordination with Cancer Treatment
- Fertility preservation procedures, such as egg or sperm freezing, typically require careful timing and coordination with the cancer treatment schedule. Early consultation ensures that there is enough time to complete these procedures before chemotherapy begins. Fertility specialists can work closely with oncologists to minimize delays in cancer treatment while still preserving the patient’s reproductive potential.
- Understanding Risks and Success Rates
- Fertility specialists can explain the risks associated with different fertility preservation methods, including the potential impact of chemotherapy on future fertility. They can also provide information on the success rates of these methods, helping patients set realistic expectations and make informed decisions.
- Emotional and Psychological Support
- The prospect of infertility can be emotionally challenging, particularly for young patients or those who have always dreamed of starting a family. Fertility specialists can offer counseling and support to help patients cope with these emotions and make decisions that align with their values and goals.
Consulting with fertility specialists before starting chemotherapy is a crucial step for patients who wish to preserve their fertility. By asking these critical questions, patients can gain a clear understanding of their options and make informed decisions that align with their personal values and future goals. Early and thorough consultation not only helps preserve the possibility of parenthood but also provides emotional support and peace of mind as patients embark on their cancer treatment journey.
The intersection of oncology and reproductive medicine has seen significant advancements in recent years, offering hope to cancer patients concerned about the impact of chemotherapy on their fertility. Researchers and clinicians have been working to develop new techniques and treatments that not only improve cancer outcomes but also minimize the risk of infertility. This section explores the latest advancements in fertility preservation and cancer treatment, highlighting promising new approaches that may reduce the impact on fertility.
13-A. Advances in Fertility Preservation Techniques
Fertility preservation has traditionally relied on methods like egg, sperm, and embryo freezing. However, recent research has led to the development of new techniques that expand the options available to patients and improve the chances of preserving fertility.
- Ovarian Tissue Cryopreservation and Re-implantation
- Background: Ovarian tissue cryopreservation involves surgically removing and freezing ovarian tissue before chemotherapy. The tissue, which contains immature eggs, can be re-implanted after cancer treatment to restore ovarian function and potentially allow for natural conception.
- Recent Advances: While ovarian tissue freezing has been considered experimental for many years, it is now becoming a more widely accepted practice, especially for young women and girls who cannot undergo egg retrieval due to time constraints or age. Recent advancements in surgical techniques and cryopreservation methods have improved the viability of the tissue after thawing. Successful pregnancies and births have been reported using re-implanted ovarian tissue, offering new hope for women who might otherwise face permanent infertility.
- Future Directions: Researchers are working to optimize the re-implantation process to increase the success rates of restoring fertility. Studies are also exploring the potential of in vitro maturation (IVM) of eggs from the thawed ovarian tissue, which could further expand fertility options.
- In Vitro Maturation (IVM)
- Background: In vitro maturation (IVM) is a technique where immature eggs are retrieved from the ovaries and matured in a laboratory setting before being fertilized or frozen. This approach can be particularly useful for women who need to begin cancer treatment immediately and do not have time for the hormonal stimulation required in traditional egg retrieval.
- Recent Advances: Recent improvements in IVM protocols have increased eggs’ maturation and fertilization rates, making it a more viable option for fertility preservation. Researchers are also exploring ways to combine IVM with other fertility preservation methods, such as ovarian tissue cryopreservation, to offer a more comprehensive approach.
- Future Directions: Continued research is focused on refining IVM techniques to improve the quality of eggs matured in vitro. This includes optimizing the culture conditions and identifying biomarkers that predict successful maturation and fertilization outcomes.
- Testicular Tissue Cryopreservation
- Background: Testicular tissue cryopreservation is an emerging technique that offers fertility preservation options for prepubescent boys who are not yet producing sperm. The tissue, which contains spermatogonial stem cells, can potentially be used in the future to restore fertility.
- Recent Advances: Although still experimental, there have been promising developments in the ability to culture and mature spermatogonial stem cells from frozen testicular tissue. Researchers are also exploring the possibility of transplanting these cells back into the testes to restore sperm production.
- Future Directions: The primary focus of ongoing research is to develop reliable methods for maturing spermatogonial stem cells into fully functional sperm that can be used for reproduction. Additionally, advancements in gene editing and regenerative medicine may offer new ways to enhance the success of this technique.
13-B. Advances in Cancer Treatments That Preserve Fertility
In addition to improvements in fertility preservation techniques, there have been significant advancements in cancer treatments designed to reduce their impact on fertility. These innovations aim to effectively treat cancer while minimizing damage to the reproductive organs.
- Targeted Therapies and Immunotherapies
- Background: Traditional chemotherapy is known for its broad-spectrum attack on rapidly dividing cells, which can lead to significant collateral damage to the reproductive system. Targeted therapies and immunotherapies, on the other hand, are designed to specifically target cancer cells while sparing healthy tissues.
- Recent Advances: Targeted therapies, such as tyrosine kinase inhibitors (TKIs) and monoclonal antibodies, have shown promise in treating certain types of cancer with fewer side effects, including less impact on fertility. Immunotherapies, which harness the body’s immune system to fight cancer, also offer a fertility-friendly alternative to traditional chemotherapy, although more research is needed to fully understand their long-term effects on reproductive health.
- Future Directions: As targeted therapies and immunotherapies continue to evolve, researchers are focused on identifying biomarkers that can predict which patients are most likely to benefit from these treatments with minimal impact on fertility. Additionally, combining these therapies with traditional treatments in a way that preserves fertility is an area of active investigation.
- Fertility-Sparing Surgical Techniques
- Background: For certain cancers, surgery is a primary treatment option. However, traditional surgical approaches often involve the removal of reproductive organs, leading to immediate infertility. Advances in fertility-sparing surgical techniques aim to treat cancer effectively while preserving as much reproductive function as possible.
- Recent Advances: In gynecological cancers such as early-stage cervical or ovarian cancer, fertility-sparing surgery—such as trachelectomy (removal of the cervix while preserving the uterus) or ovarian transposition (moving the ovaries out of the radiation field)—has become more common. These techniques allow women to maintain their reproductive organs and potentially conceive naturally after treatment.
- Future Directions: Researchers are working to refine these surgical techniques and develop new approaches that further minimize the impact on fertility. The use of robotics and minimally invasive surgery is also being explored to improve outcomes and reduce recovery times.
- Radiation Therapy Innovations
- Background: Radiation therapy, especially when directed at the pelvic area, can have devastating effects on fertility by damaging the ovaries, testes, or the uterus. Innovations in radiation therapy aim to target cancerous tissues more precisely, thereby reducing exposure to healthy reproductive organs.
- Recent Advances: Techniques such as intensity-modulated radiation therapy (IMRT) and proton therapy have been developed to deliver high doses of radiation directly to the tumor while sparing surrounding healthy tissue. Additionally, ovarian transposition, where the ovaries are surgically moved out of the radiation field, has proven effective in preserving ovarian function.
- Future Directions: The future of radiation therapy lies in the continued refinement of these precision techniques, along with the development of new technologies that further limit exposure to reproductive organs. Research is also ongoing into the use of radioprotective agents that could shield reproductive tissues from the harmful effects of radiation.
13-C. Emerging Technologies and Future Research
The field of fertility preservation and cancer treatment is continually evolving, with new technologies and research paving the way for even better outcomes for cancer patients who wish to retain their fertility.
- Gene Editing and Regenerative Medicine:
- Background: Advances in gene editing technologies, such as CRISPR-Cas9, have opened new possibilities for treating cancer and preserving fertility. Regenerative medicine, which focuses on repairing or replacing damaged tissues, also holds promise for restoring fertility after cancer treatment.
- Recent Advances: Researchers are exploring the potential of using gene editing to repair DNA damage in reproductive cells caused by chemotherapy. Additionally, regenerative medicine techniques, such as stem cell therapy, are being investigated as a way to regenerate ovarian or testicular tissue damaged by cancer treatment.
- Future Directions: The combination of gene editing and regenerative medicine could revolutionize fertility preservation by offering new ways to protect and restore reproductive function. Clinical trials are needed to evaluate the safety and efficacy of these approaches in cancer patients.
- Artificial Ovaries and Testes:
- Background: The concept of artificial ovaries and testes involves creating bioengineered organs that can produce eggs or sperm. This could provide a solution for patients who have lost their reproductive organs due to cancer treatment.
- Recent Advances: Early research has demonstrated the feasibility of creating artificial ovaries that can support the growth and maturation of eggs. Similarly, studies are underway to develop artificial testes capable of producing viable sperm.
- Future Directions: While still in the experimental stages, artificial ovaries and testes could eventually offer a viable fertility preservation option for cancer patients. Further research is needed to refine these technologies and bring them closer to clinical use.
- Cryopreservation of Organoids
- Background: Organoids are miniature, simplified versions of organs grown in vitro from stem cells. The cryopreservation of organoids offers a potential new method for preserving fertility by freezing these complex structures, which could be used to restore reproductive function later.
- Recent Advances: Researchers have successfully created and cryopreserved organoids of various organs, including the ovaries and testes. These organoids have the potential to be thawed and used to restore reproductive function in patients who have lost fertility due to cancer treatment.
- Future Directions: The application of organoid technology in fertility preservation is still in its infancy, but it holds great promise. Future research will focus on improving the viability and functionality of these organoids after thawing, as well as exploring their use in human fertility preservation.
The latest advancements in fertility preservation and cancer treatment offer new hope to patients who face the dual challenge of fighting cancer and preserving their ability to have children. As research continues to progress, these innovations will likely become more widely available, improving outcomes and expanding options for cancer patients. By staying informed about these developments, patients and healthcare providers can work together to make decisions that align with the
Conclusion
The intersection of chemotherapy and fertility is a critical and deeply personal issue for cancer patients, particularly those of reproductive age who hope to have biological children in the future. Chemotherapy, while an essential and often life-saving treatment, poses significant risks to fertility in both men and women. These risks vary depending on factors such as the type of cancer, the specific chemotherapy regimen, the patient’s age, and overall reproductive health. However, advances in fertility preservation techniques and cancer treatment offer hope and options for patients facing these challenges.
Throughout this article, we have explored the multifaceted impact of chemotherapy on fertility, delving into the biological mechanisms that cause infertility, the psychological toll on patients, and the various strategies available to preserve fertility before treatment begins. Key fertility preservation methods, such as egg and sperm freezing, embryo cryopreservation, and newer approaches like ovarian and testicular tissue freezing, provide valuable options for patients who want to safeguard their reproductive potential. We also discussed recent advancements in research and treatments that aim to reduce the impact of chemotherapy on fertility, including targeted therapies, fertility-sparing surgeries, and innovative technologies like gene editing and artificial reproductive organs.
The importance of early intervention and informed decision-making cannot be overstated. Patients diagnosed with cancer should be encouraged to consult with fertility specialists as soon as possible to explore their options and develop a personalized fertility preservation plan. This proactive approach ensures that patients have the best possible chance of preserving their fertility before starting chemotherapy. It also provides emotional and psychological reassurance, helping patients feel more in control of their future during a challenging and uncertain time.
In summary, while chemotherapy-induced infertility is a serious concern, the advancements in fertility preservation and cancer treatment provide a pathway for many patients to achieve their dreams of parenthood. Early consultation, informed choices, and access to the latest medical innovations are essential in navigating this complex journey. With the right support and resources, patients can face the future with hope and confidence, knowing that they have taken steps to protect their ability to build a family after cancer.
Frequently Asked Questions (FAQs)
How does chemotherapy affect fertility?
- Chemotherapy can damage reproductive organs and reduce the ability to produce eggs or sperm, leading to temporary or permanent infertility.
What are the chances of fertility returning after chemotherapy?
- The chances vary depending on the patient’s age, the type and dosage of chemotherapy drugs used, and individual health factors.
Can I get pregnant after chemotherapy?
- Pregnancy after chemotherapy is possible for some individuals, but it often depends on whether fertility was preserved before treatment and how the body recovers post-treatment.
How can I preserve my fertility before chemotherapy?
- Options include egg or sperm freezing, ovarian or testicular tissue preservation, and newer fertility preservation techniques.
What psychological support is available for those who have experienced chemotherapy-induced infertility?
- Counseling, support groups, and therapy are available to help individuals cope with the emotional and psychological impacts of infertility.
What are the latest advancements in fertility preservation?
- Recent advances include improved cryopreservation techniques, ovarian and testicular tissue freezing, and research into less harmful cancer treatments that preserve fertility.